Englitazone administration to late pregnant rats produces delayed body growth and insulin resistance in their fetuses and neonates
- PMID: 15810879
- PMCID: PMC1180742
- DOI: 10.1042/BJ20041837
Englitazone administration to late pregnant rats produces delayed body growth and insulin resistance in their fetuses and neonates
Abstract
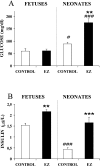
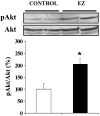
The level of maternal circulating triacylglycerols during late pregnancy has been correlated with the mass of newborns. PPARgamma (peroxisome-proliferator-activated receptor gamma) ligands, such as TZDs (thiazolidinediones), have been shown to reduce triacylglycerolaemia and have also been implicated in the inhibition of tissue growth and the promotion of cell differentiation. Therefore TZDs might control cell proliferation during late fetal development and, by extension, body mass of pups. To investigate the response to EZ (englitazone), a TZD, on perinatal development, 0 or 50 mg of englitazone/kg of body mass was given as an oral dose to pregnant rats daily from day 16 of gestation until either day 20 for the study of their fetuses, or until day 21 of gestation for the study of neonates. EZ decreased maternal triacylglycerol levels at day 20 of gestation and neonatal mass, but not fetal mass. Fetuses and neonates from EZ-treated mothers exhibited high levels of insulin and were found to be hyperglycaemic. The apparent insulin-resistant state in neonates from EZ-treated pregnant rats was corroborated, since they showed higher plasma NEFA [non-esterified ('free') fatty acid] levels, ketonaemia and liver LPL (lipoprotein lipase) activity and lower plasma IGF-I (type 1 insulin-like growth factor) levels, in comparison with those from control mothers. Moreover, at the molecular level, an increase in Akt phosphorylation was found in the liver of neonates from EZ-treated mothers, which confirms that the insulin pathway was negatively affected. Thus the response of fetuses and neonates to maternal antidiabetic drug treatment is the opposite of what would be expected, and can be justified by the scarce amount of adipose tissue impeding a normal response to PPARgamma ligands and by hyperinsulinaemia as being responsible for a major insulin-resistant condition.
Figures
Similar articles
-
Different responses to maternal diabetes during the first and second half of gestation in the streptozotocin-treated rat.Isr J Med Sci. 1991 Aug-Sep;27(8-9):442-8. Isr J Med Sci. 1991. PMID: 1835719
-
Effects of maternal protein malnutrition on fetal growth, plasma insulin-like growth factors, insulin-like growth factor binding proteins, and liver insulin-like growth factor gene expression in the rat.Pediatr Res. 1995 Mar;37(3):334-42. doi: 10.1203/00006450-199503000-00014. Pediatr Res. 1995. PMID: 7540281
-
NTP technical report on the toxicity studies of Dibutyl Phthalate (CAS No. 84-74-2) Administered in Feed to F344/N Rats and B6C3F1 Mice.Toxic Rep Ser. 1995 Apr;30:1-G5. Toxic Rep Ser. 1995. PMID: 12209194
-
Maternal taurine supplementation in the late pregnant rat stimulates postnatal growth and induces obesity and insulin resistance in adult offspring.J Physiol. 2007 Mar 15;579(Pt 3):823-33. doi: 10.1113/jphysiol.2006.124610. Epub 2007 Jan 4. J Physiol. 2007. PMID: 17204495 Free PMC article.
-
Late but not early gestational maternal growth hormone treatment increases fetal adiposity in overnourished adolescent sheep.Biol Reprod. 2006 Aug;75(2):231-9. doi: 10.1095/biolreprod.106.052605. Epub 2006 May 10. Biol Reprod. 2006. PMID: 16687645
Cited by
-
Oral Hypoglycemic Agents in pregnancy: An Update.J Obstet Gynaecol India. 2013 Apr;63(2):82-7. doi: 10.1007/s13224-012-0312-z. Epub 2013 Mar 27. J Obstet Gynaecol India. 2013. PMID: 24431611 Free PMC article. Review.
-
Thiazolidinediones and Fertility in Polycystic Ovary Syndrome (PCOS).PPAR Res. 2006;2006:73986. doi: 10.1155/PPAR/2006/73986. PPAR Res. 2006. PMID: 17347533 Free PMC article.
-
Childhood obesity and environmental chemicals.Mt Sinai J Med. 2011 Jan-Feb;78(1):22-48. doi: 10.1002/msj.20229. Mt Sinai J Med. 2011. PMID: 21259261 Free PMC article. Review.
-
Connecting Metainflammation and Neuroinflammation Through the PTN-MK-RPTPβ/ζ Axis: Relevance in Therapeutic Development.Front Pharmacol. 2019 Apr 12;10:377. doi: 10.3389/fphar.2019.00377. eCollection 2019. Front Pharmacol. 2019. PMID: 31031625 Free PMC article. Review.
-
Pioglitazone therapy in mouse offspring exposed to maternal obesity.Am J Obstet Gynecol. 2013 Apr;208(4):308.e1-7. doi: 10.1016/j.ajog.2013.01.013. Epub 2013 Jan 10. Am J Obstet Gynecol. 2013. PMID: 23313309 Free PMC article.
References
-
- Bocos C., Gottlicher M., Gearing K., Banner C., Enmark E., Teboul M., Crickmore A., Gustafson J.-Å. Fatty acid activation of peroxisome proliferator-activated receptor (PPAR) J. Steroid Biochem. Mol. Biol. 1995;53:467–473. - PubMed
-
- Zilversmit D. B. Atherogenic nature of triglycerides, postprandial lipidemia, and triglyceride-rich remnant lipoproteins. Clin. Chem. 1995;41:153–158. - PubMed
-
- Watts G. F., Dimmitt S. B. Fibrates, dyslipoproteinaemia and cardiovascular disease. Curr. Opin. Lipidol. 1999;10:561–574. - PubMed
-
- Lefebvre A. M., Peinado-Onsurbe J., Leitersdorf I., Briggs M. R., Paterniti J. R., Fruchart J. C., Fievet C., Auwerx J., Staels B. Regulation of lipoprotein metabolism by thiazolidinediones occurs through a distinct but complementary mechanism relative to fibrates. Arterioscler. Thromb. Vasc. Biol. 1997;17:1756–1764. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

