Direct microbial reduction and subsequent preservation of uranium in natural near-surface sediment
- PMID: 15812002
- PMCID: PMC1082503
- DOI: 10.1128/AEM.71.4.1790-1797.2005
Direct microbial reduction and subsequent preservation of uranium in natural near-surface sediment
Abstract
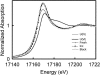
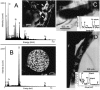
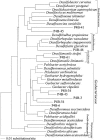
The fate of uranium in natural systems is of great environmental importance. X-ray absorption near-edge spectroscopy (XANES) revealed that U(VI) was reduced to U(IV) in shallow freshwater sediment at an open pit in an inactive uranium mine. Geochemical characterization of the sediment showed that nitrate, Fe(III), and sulfate had also been reduced in the sediment. Observations of the sediment particles and microbial cells by scanning and transmission electron microscopy, coupled with elemental analysis by energy dispersive spectroscopy, revealed that uranium was concentrated at microbial cell surfaces. U(IV) was not associated with framboidal pyrite or nanometer-scale iron sulfides, which are presumed to be of microbial origin. Uranium concentrations were not detected in association with algal cells. Phylogenetic analyses of microbial populations in the sediment by the use of 16S rRNA and dissimilatory sulfite reductase gene sequences detected organisms belonging to the families Geobacteraceae and Desulfovibrionaceae. Cultivated members of these lineages reduce U(VI) and precipitate iron sulfides. The association of uranium with cells, but not with sulfide surfaces, suggests that U(VI) is reduced by the enzymatic activities of microorganisms. Uranium was highly enriched (760 ppm) in a subsurface black layer in unsaturated sediment sampled from a pit which was exposed to seasonal fluctuations in the pond level. XANES analysis showed that the majority of uranium in this layer was U(IV), indicating that uranium is preserved in its reduced form after burial.
Figures

References
-
- Anderson, R. T., H. A. Vrionis, I. Ortiz-Bernad, C. T. Resch, P. E. Long, R. Dayvault, K. Karp, S. Marutzky, D. R. Metzler, A. Peacock, D. C. White, M. Lowe, and D. R. Lovley. 2003. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microbiol. 69:5884-5891. - PMC - PubMed
-
- Barnes, C. E., and J. K. Cochran. 1993. Uranium geochemistry in estuarine sediments: controls on removal and release processes. Geochim. Cosmochim. Acta 57:555-569.
-
- Berner, R. A. 1982. Burial of organic carbon and pyrite sulfur in the modern ocean: its geochemical and environmental significance. Am. J. Sci. 282:451-473.
-
- Cross, J. L., and A. I. Frenkel. 1998. Use of scattered radiation for absolute energy calibration. Rev. Sci. Instrum. 70:38-40.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

