Xanthomonas axonopodis pv. phaseoli var. fuscans is aggregated in stable biofilm population sizes in the phyllosphere of field-grown beans
- PMID: 15812033
- PMCID: PMC1082538
- DOI: 10.1128/AEM.71.4.2008-2015.2005
Xanthomonas axonopodis pv. phaseoli var. fuscans is aggregated in stable biofilm population sizes in the phyllosphere of field-grown beans
Abstract
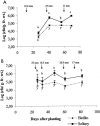
The occurrence of "Xanthomonas axonopodis pv. phaseoli var. fuscans" (proposed name) populations as biofilms on bean leaves was investigated during three field experiments on plots established with naturally contaminated bean seeds. Behavior of aggregated versus solitary populations was determined by quantification of culturable cells in different fractions of the epiphytic population separated by particle size. X. axonopodis pv. phaseoli var. fuscans population dynamic studies confirmed an asymptomatic and epiphytic colonization of the bean phyllosphere. For all years of experiment and cultivars tested, biofilms and solitary components of the populations were always detected. Biofilm population sizes remained stable throughout the growing season (around 10(5) CFU/g of fresh weight) while solitary population sizes were more abundant and varied with climate. According to enterobacterial repetitive intergenic consensus fingerprinting, aggregated bacterial isolates were not different from solitary isolates. In controlled conditions, application of a hydric stress resulted in a decrease of the solitary populations on the leaf surface while the biofilm fraction remained stable. Suppression of the hydric stress allowed solitary bacterial populations to increase again. Aggregation in biofilms on leaf surfaces provides protection to the bacterial cells against hydric stress.
Figures


References
-
- Barber, C. E., J. L. Tang, J. X. Feng, M. Q. Pan, T. J. G. Wilson, H. Slater, J. M. Dow, P. Williams, and M. J. Daniels. 1997. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol. Microbiol. 24:555-566. - PubMed
-
- Boureau, T., M.-A. Jacques, R. Berruyer, Y. Dessaux, H. Dominguez, and C. E. Morris. 2004. Comparison of the phenotypes and genotypes of biofilm and solitary epiphytic bacterial populations on broad-leaved endive. Microb. Ecol. 47:87-95. - PubMed
-
- Broughton, W. J., G. Hernandez, M. Blair, S. Beebe, P. Gepts, and J. Vanderleyden. 2003. Beans (Phaseolus spp.)—model food legumes. Plant Soil 252:55-128.
-
- Carmichael, I., I. S. Harper, M. J. Coventry, P. W. J. Taylor, J. Wan, and M. W. Hickey. 1999. Bacterial colonization and biofilm development on minimally processed vegetables. J. Appl. Microbiol. Symp. Suppl. 85:45S-51S. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

