Anaerobic, nitrate-dependent oxidation of U(IV) oxide minerals by the chemolithoautotrophic bacterium Thiobacillus denitrificans
- PMID: 15812053
- PMCID: PMC1082518
- DOI: 10.1128/AEM.71.4.2170-2174.2005
Anaerobic, nitrate-dependent oxidation of U(IV) oxide minerals by the chemolithoautotrophic bacterium Thiobacillus denitrificans
Abstract
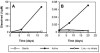
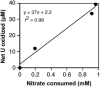
Under anaerobic conditions and at circumneutral pH, cells of the widely distributed, obligate chemolithoautotrophic bacterium Thiobacillus denitrificans oxidatively dissolved synthetic and biogenic U(IV) oxides (uraninite) in nitrate-dependent fashion: U(IV) oxidation required the presence of nitrate and was strongly correlated with nitrate consumption. This is the first report of anaerobic U(IV) oxidation by an autotrophic bacterium.
Figures

References
-
- Brina, R., and A. G. Miller. 1992. Direct detection of trace levels of uranium by laser-induced kinetic phosphorimetry. Anal. Chem. 64:1413-1418.
-
- DiSpirito, A. A., and O. H. Tuovinen. 1982. Uranous ion oxidation and carbon dioxide fixation by Thiobacillus ferrooxidans. Arch. Microbiol. 133:28-32.
-
- Finneran, K. T., M. E. Housewright, and D. R. Lovley. 2002. Multiple influences of nitrate on uranium solubility during bioremediation of uranium-contaminated subsurface sediments. Environ. Microbiol. 4:510-516. - PubMed
-
- Fredrickson, J. K., J. M. Zachara, D. W. Kennedy, M. C. Duff, Y. A. Gorby, S. W. Li, and K. M. Krupka. 2000. Reduction of U(VI) in goethite (α-FeOOH) suspensions by a dissimilatory metal-reducing bacterium. Geochim. Cosmochim. Acta 64:3085-3098.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

