Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans
- PMID: 15812057
- PMCID: PMC1082548
- DOI: 10.1128/AEM.71.4.2186-2189.2005
Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans
Abstract
In experiments performed using graphite electrodes poised by a potentiostat (+200 mV versus Ag/AgCl) or in a microbial fuel cell (with oxygen as the electron acceptor), the Fe(III)-reducing organism Geothrix fermentans conserved energy to support growth by coupling the complete oxidation of acetate to reduction of a graphite electrode. Other organic compounds, such as lactate, malate, propionate, and succinate as well as components of peptone and yeast extract, were utilized for electricity production. However, electrical characteristics and the results of shuttling assays indicated that unlike previously described electrode-reducing microorganisms, G. fermentans produced a compound that promoted electrode reduction. This is the first report of complete oxidation of organic compounds linked to electrode reduction by an isolate outside of the Proteobacteria.
Figures


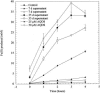
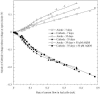
References
-
- Bond, D. R., D. E. Holmes, L. M. Tender, and D. R. Lovley. 2002. Electrode-reducing microorganisms harvesting energy from marine sediments. Science 295:483-485. - PubMed
-
- Chaudhuri, S. K., and D. R. Lovley. 2003. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 21:1229-1232. - PubMed
-
- Childers, S. E., S. Ciufo, and D. R. Lovley. 2002. Geobacter metallireducens accesses insoluble Fe(III) oxide by chemotaxis. Nature 416:767-769. - PubMed
-
- Choi, Y., N. Kim, S. Kim, and S. Jung. 2003. Dynamic behaviors of redox mediators within the hydrophobic layers as an important factor for effective microbial fuel cell operation. Bull. Korean Chem. Soc. 24:437-440.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

