New players tip the scales in the balance between excitatory and inhibitory synapses
- PMID: 15813960
- PMCID: PMC1079938
- DOI: 10.1186/1744-8069-1-12
New players tip the scales in the balance between excitatory and inhibitory synapses
Abstract
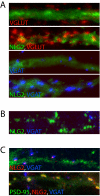
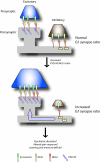
Synaptogenesis is a highly controlled process, involving a vast array of players which include cell adhesion molecules, scaffolding and signaling proteins, neurotransmitter receptors and proteins associated with the synaptic vesicle machinery. These molecules cooperate in an intricate manner on both the pre- and postsynaptic sides to orchestrate the precise assembly of neuronal contacts. This is an amazing feat considering that a single neuron receives tens of thousands of synaptic inputs but virtually no mismatch between pre- and postsynaptic components occur in vivo. One crucial aspect of synapse formation is whether a nascent synapse will develop into an excitatory or inhibitory contact. The tight control of a balance between the types of synapses formed regulates the overall neuronal excitability, and is thus critical for normal brain function and plasticity. However, little is known about how this balance is achieved. This review discusses recent findings which provide clues to how neurons may control excitatory and inhibitory synapse formation, with focus on the involvement of the neuroligin family and PSD-95 in this process.
Figures
Similar articles
-
Overexpression of the cell adhesion protein neuroligin-1 induces learning deficits and impairs synaptic plasticity by altering the ratio of excitation to inhibition in the hippocampus.Hippocampus. 2010 Feb;20(2):305-22. doi: 10.1002/hipo.20630. Hippocampus. 2010. PMID: 19437420
-
Plasticity of neuronal excitability: Hebbian rules beyond the synapse.Arch Ital Biol. 2007 Nov;145(3-4):277-87. Arch Ital Biol. 2007. PMID: 18075121 Review.
-
Molecular dynamics of postsynaptic receptors and scaffold proteins.Curr Opin Neurobiol. 2008 Oct;18(5):532-40. doi: 10.1016/j.conb.2008.09.009. Epub 2008 Oct 23. Curr Opin Neurobiol. 2008. PMID: 18832033 Review.
-
Matching of pre- and postsynaptic specializations during synaptogenesis.Neuroscientist. 2007 Apr;13(2):115-26. doi: 10.1177/1073858406296803. Neuroscientist. 2007. PMID: 17404372 Review.
-
Astrocyte-derived estrogen enhances synapse formation and synaptic transmission between cultured neonatal rat cortical neurons.Neuroscience. 2007 Feb 23;144(4):1229-40. doi: 10.1016/j.neuroscience.2006.09.056. Epub 2006 Dec 19. Neuroscience. 2007. PMID: 17184929
Cited by
-
Research on the traditional Chinese medicine treating gastrointestinal motility in diabetic rats by improving biomechanical remodeling and neuroendocrine regulation.Am J Transl Res. 2017 May 15;9(5):2219-2230. eCollection 2017. Am J Transl Res. 2017. PMID: 28559973 Free PMC article.
-
Protein tyrosine phosphatases PTPδ, PTPσ, and LAR: presynaptic hubs for synapse organization.Trends Neurosci. 2013 Sep;36(9):522-34. doi: 10.1016/j.tins.2013.06.002. Epub 2013 Jul 5. Trends Neurosci. 2013. PMID: 23835198 Free PMC article. Review.
-
Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2.Neuron. 2007 Jun 21;54(6):919-31. doi: 10.1016/j.neuron.2007.05.029. Neuron. 2007. PMID: 17582332 Free PMC article.
-
Neuroligin-2 deletion selectively decreases inhibitory synaptic transmission originating from fast-spiking but not from somatostatin-positive interneurons.J Neurosci. 2009 Nov 4;29(44):13883-97. doi: 10.1523/JNEUROSCI.2457-09.2009. J Neurosci. 2009. PMID: 19889999 Free PMC article.
-
Excitation Control: Balancing PSD-95 Function at the Synapse.Front Mol Neurosci. 2008 Mar 28;1:4. doi: 10.3389/neuro.02.004.2008. eCollection 2008. Front Mol Neurosci. 2008. PMID: 18946537 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

