Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation
- PMID: 15813963
- PMCID: PMC1083421
- DOI: 10.1186/1744-8069-1-13
Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation
Abstract
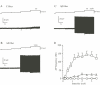
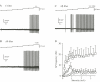
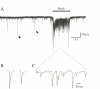
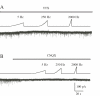
Transcutaneous sine-wave stimuli at frequencies of 2000, 250 and 5 Hz (Neurometer) are thought to selectively activate Abeta, Adelta and C afferent fibers, respectively. However, there are few reports to test the selectivity of these stimuli at the cellular level. In the present study, we analyzed action potentials (APs) generated by sine-wave stimuli applied to the dorsal root in acutely isolated rat dorsal root ganglion (DRG) preparations using intracellular recordings. We also measured excitatory synaptic responses evoked by transcutaneous stimuli in substantia gelatinosa (SG) neurons of the spinal dorsal horn, which receive inputs predominantly from C and Adelta fibers, using in vivo patch-clamp recordings. In behavioral studies, escape or vocalization behavior of rats was observed with both 250 and 5 Hz stimuli at intensity of approximately 0.8 mA (T5/ T250), whereas with 2000 Hz stimulation, much higher intensity (2.14 mA, T2000) was required. In DRG neurons, APs were generated at T5/T250 by 2000 Hz stimulation in Abeta, by 250 Hz stimulation both in Abeta and Adelta, and by 5 Hz stimulation in all three classes of DRG neurons. However, the AP frequencies elicited in Abeta and Adelta by 5 Hz stimulation were much less than those reported previously in physiological condition. With in vivo experiments large amplitude of EPSCs in SG neurons were elicited by 250 and 5 Hz stimuli at T5/ T250. These results suggest that 2000 Hz stimulation excites selectively Abeta fibers and 5 Hz stimulation activates noxious transmission mediated mainly through C fibers. Although 250 Hz stimulation activates both Adelta and Abeta fibers, tactile sensation would not be perceived when painful sensation is produced at the same time. Therefore, 250 Hz was effective stimulus frequency for activation of Adelta fibers initiating noxious sensation. Thus, the transcutaneous sine-wave stimulation can be applied to evaluate functional changes of sensory transmission by comparing thresholds with the three stimulus frequencies.
Figures


Similar articles
-
Alteration in synaptic inputs through C-afferent fibers to substantia gelatinosa neurons of the rat spinal dorsal horn during postnatal development.Neuroscience. 2000;99(3):549-56. doi: 10.1016/s0306-4522(00)00224-4. Neuroscience. 2000. PMID: 11029546
-
Reorganization of the primary afferent termination in the rat spinal dorsal horn during post-natal development.Brain Res Dev Brain Res. 1999 Mar 12;113(1-2):29-36. doi: 10.1016/s0165-3806(98)00186-2. Brain Res Dev Brain Res. 1999. PMID: 10064871
-
Actions of propofol on substantia gelatinosa neurones in rat spinal cord revealed by in vitro and in vivo patch-clamp recordings.Eur J Neurosci. 2009 Feb;29(3):518-28. doi: 10.1111/j.1460-9568.2008.06607.x. Eur J Neurosci. 2009. PMID: 19222560
-
Mechanisms for the Clinical Utility of Low-Frequency Stimulation in Neuromodulation of the Dorsal Root Ganglion.Neuromodulation. 2021 Jun;24(4):738-745. doi: 10.1111/ner.13323. Epub 2020 Nov 25. Neuromodulation. 2021. PMID: 33236811 Review.
-
Mechanisms of Dorsal Root Ganglion Stimulation in Pain Suppression: A Computational Modeling Analysis.Neuromodulation. 2018 Apr;21(3):234-246. doi: 10.1111/ner.12754. Epub 2018 Jan 29. Neuromodulation. 2018. PMID: 29377442 Review.
Cited by
-
Both ipsilateral and contralateral localized vibratory stimulations modulated pain-related sensory thresholds on the foot in mice and humans.J Pain Res. 2018 Aug 28;11:1645-1657. doi: 10.2147/JPR.S162379. eCollection 2018. J Pain Res. 2018. PMID: 30214274 Free PMC article.
-
Pharmacological switch in Abeta-fiber stimulation-induced spinal transmission in mice with partial sciatic nerve injury.Mol Pain. 2008 Jul 11;4:25. doi: 10.1186/1744-8069-4-25. Mol Pain. 2008. PMID: 18620588 Free PMC article.
-
Bortezomib-induced painful peripheral neuropathy: an electrophysiological, behavioral, morphological and mechanistic study in the mouse.PLoS One. 2013 Sep 12;8(9):e72995. doi: 10.1371/journal.pone.0072995. eCollection 2013. PLoS One. 2013. PMID: 24069168 Free PMC article.
-
Supportive effect of interferential current stimulation on susceptibility of swallowing in guinea pigs.Exp Brain Res. 2018 Oct;236(10):2661-2676. doi: 10.1007/s00221-018-5325-0. Epub 2018 Jul 4. Exp Brain Res. 2018. PMID: 29974148
-
Neonatal pain: What's age got to do with it?Surg Neurol Int. 2014 Nov 13;5(Suppl 13):S479-89. doi: 10.4103/2152-7806.144630. eCollection 2014. Surg Neurol Int. 2014. PMID: 25506507 Free PMC article.
References
-
- Dinh S, Marroquin E, Raj PP. Neuroselective quantification of allodynia by current perception threshold evaluation on CRPS (RSD) patients. Reg Anesth. 1997;22:44.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

