A functional role for small-conductance calcium-activated potassium channels in sensory pathways including nociceptive processes
- PMID: 15814779
- PMCID: PMC6725366
- DOI: 10.1523/JNEUROSCI.0597-05.2005
A functional role for small-conductance calcium-activated potassium channels in sensory pathways including nociceptive processes
Abstract
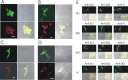
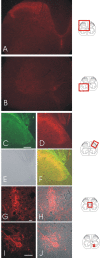
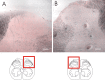
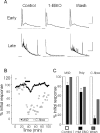
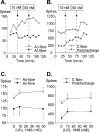
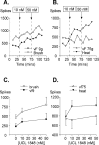
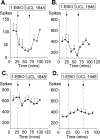
We investigated the role of small-conductance calcium-activated potassium (SK) and intermediate-conductance calcium-activated potassium channels in modulating sensory transmission from peripheral afferents into the rat spinal cord. Subunit-specific antibodies reveal high levels of SK3 immunoreactivity in laminas I, II, and III of the spinal cord. Among dorsal root ganglion neurons, both peripherin-positive (C-type) and peripherin-negative (A-type) cells show intense SK3 immunoreactivity. Furthermore, dorsal root-stimulated sensory responses recorded in vitro are inhibited when SK channel activity is increased with 1-ethyl-2-benzimidazolinone (1-EBIO). In vivo electrophysiological recordings show that neuronal responses to naturally evoked nociceptive and nonnociceptive stimuli increase after application of the selective SK channel blocker 8,14-diaza-1,7(1,4)-diquinolinacyclotetradecaphanedium di-trifluoroacetate (UCL 1848), indicating that SK channels are normally active in moderating afferent input. Conversely, neuronal responses evoked by mechanical stimuli are inhibited when SK channel activity is increased with 1-EBIO. These effects are reversed by the subsequent application of UCL 1848. Our data demonstrate that SK channels have an important role in controlling sensory input into the spinal cord.
Figures


References
-
- Abdulla FA, Smith PA (2001) Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. J Neurophysiol 85: 644-658. - PubMed
-
- Banks BE, Brown C, Burgess GM, Burnstock G, Claret M, Cocks TM, Jenkinson DH (1979) Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature 282: 415-417. - PubMed
-
- Blank T, Nijholt I, Kye MJ, Radulovic J, Spiess J (2003) Small-conductance, Ca2+-activated K+ channel SK3 generates age-related memory and LTP deficits. Nat Neurosci 6: 911-912. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
