Robustness of burst firing in dissociated purkinje neurons with acute or long-term reductions in sodium conductance
- PMID: 15814781
- PMCID: PMC6725377
- DOI: 10.1523/JNEUROSCI.3929-04.2005
Robustness of burst firing in dissociated purkinje neurons with acute or long-term reductions in sodium conductance
Abstract
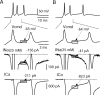
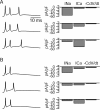
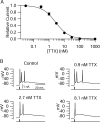
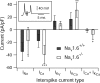
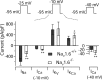
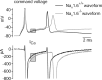
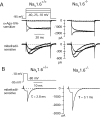
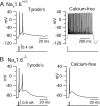
Cerebellar Purkinje neurons often generate all-or-none burst firing in response to depolarizing stimuli. Voltage-clamp experiments using action potential waveforms show that burst firing depends on small net inward currents that flow after spikes and reflect the net balance between multiple large currents. Given this, burst firing is surprisingly robust in the face of changes in the magnitude of the underlying currents from cell to cell. We explored the basis of this robustness by examining the effects of reducing the sodium current, the major contributor to the postspike inward current. Burst firing persisted in concentrations of tetrodotoxin that produced half-block of sodium current. This robustness of bursting reflects an acute feedback mechanism whereby waveform changes from the reduced sodium current (reduced spike height and a hyperpolarizing shift in postspike voltage) cause compensatory decreases in postspike potassium currents. In particular, reduced spike height reduces calcium entry and subsequent calcium-activated potassium current, and the hyperpolarizing shift in postspike voltage speeds deactivation of Kv3-like potassium channels. Other experiments examined bursting in Na(v)1.6-/- mice, in which sodium current density is reduced in the long term. Under these circumstances, there was upregulation of both T-type and P-type calcium current and a change in the balance of calcium current and calcium-activated potassium current such that their net influence shifted from being inhibitory during bursts in wild-type neurons to excitatory during bursts from Na(v)1.6-/- mutant neurons. Thus, Purkinje neurons have both acute and long-term feedback mechanisms that serve to maintain burst firing when voltage-dependent sodium conductance is reduced.
Figures




References
-
- Bezprozvanny I, Tsien RW (1995) Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967). Mol Pharmacol 48: 540-549. - PubMed
-
- Callaway JC, Ross WN (1997) Spatial distribution of synaptically activated sodium concentration changes in cerebellar Purkinje neurons. J Neurophysiol 77: 145-152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
