Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids
- PMID: 15814788
- PMCID: PMC6725389
- DOI: 10.1523/JNEUROSCI.4177-04.2005
Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids
Abstract
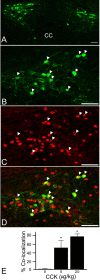
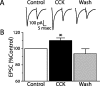
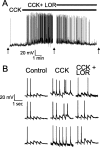
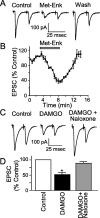
The nucleus tractus solitarius (NTS) receives dense terminations from cranial visceral afferents, including those from the gastrointestinal (GI) system. Although the NTS integrates peripheral satiety signals and relays this signal to central feeding centers, little is known about which NTS neurons are involved or what mechanisms are responsible. Proopiomelanocortin (POMC) neurons are good candidates for GI integration, because disruption of the POMC gene leads to severe obesity and hyperphagia. Here, we used POMC-enhanced green fluorescent protein (EGFP) transgenic mice to identify NTS POMC neurons. Intraperitoneal administration of cholecystokinin (CCK) induced c-fos gene expression in NTS POMC-EGFP neurons, suggesting that they are activated by afferents stimulated by the satiety hormone. We tested the synaptic relationship of these neurons to visceral afferents and their modulation by CCK and opioids using patch recordings in horizontal brain slices. Electrical activation of the solitary tract (ST) evoked EPSCs in NTS POMC-EGFP neurons. The invariant latencies, low failure rates, and substantial paired-pulse depression of the ST-evoked EPSCs indicate that NTS POMC-EGFP neurons are second-order neurons directly contacted by afferent terminals. The EPSCs were blocked by the glutamate antagonist 2,3-dihydroxy-6-nitro-7-sulfonyl-benzo[f]quinoxaline. CCK increased the amplitude of the ST-stimulated EPSCs and the frequency of miniature EPSCs, effects attenuated by the CCK1 receptor antagonist lorglumide. In contrast, the orexigenic opioid agonists [D-Ala(2), N-Me-Phe(4), Gly-ol(5)]-enkephalin and met-enkephalin inhibited both ST-stimulated EPSCs and the frequency of miniature EPSCs. These findings identify a potential satiety pathway in which visceral afferents directly activate NTS POMC-EGFP neurons with excitatory inputs that are appropriately modulated by appetite regulators.
Figures



References
-
- Aicher SA, Goldberg A, Sharma S, Pickel VM (2000) mu-opioid receptors are present in vagal afferents and their dendritic targets in the medial nucleus tractus solitarius. J Comp Neurol 422: 181-190. - PubMed
-
- Blevins JE, Stanley BG, Reidelberger RD (2000) Brain regions where cholecystokinin suppresses feeding in rats. Brain Res 860: 1-10. - PubMed
-
- Bodnar RJ, Hadjimarkou MM (2002) Endogenous opiates and behavior:2001. Peptides 23: 2307-2365. - PubMed
-
- Broberger C, Hokfelt T (2001) Hypothalamic and vagal neuropeptide circuitries regulating food intake. Physiol Behav 74: 669-682. - PubMed
-
- Broberger C, Holmberg K, Shi TJ, Dockray G, Hokfelt T (2001) Expression and regulation of cholecystokinin and cholecystokinin receptors in rat nodose and dorsal root ganglia. Brain Res 903: 128-140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
