Rapid upregulation of alpha7 nicotinic acetylcholine receptors by tyrosine dephosphorylation
- PMID: 15814802
- PMCID: PMC6725387
- DOI: 10.1523/JNEUROSCI.5389-03.2005
Rapid upregulation of alpha7 nicotinic acetylcholine receptors by tyrosine dephosphorylation
Abstract
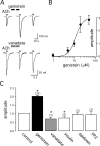
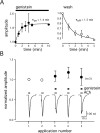
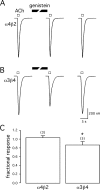
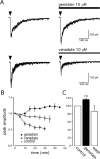
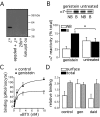
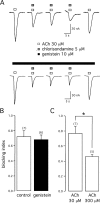
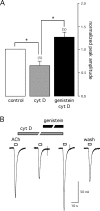
Alpha7 nicotinic acetylcholine receptors (nAChRs) modulate network activity in the CNS. Thus, functional regulation of alpha7 nAChRs could influence the flow of information through various brain nuclei. It is hypothesized here that these receptors are amenable to modulation by tyrosine phosphorylation. In both Xenopus oocytes and rat hippocampal interneurons, brief exposure to a broad-spectrum protein tyrosine kinase inhibitor, genistein, specifically and reversibly potentiated alpha7 nAChR-mediated responses, whereas a protein tyrosine phosphatase inhibitor, pervanadate, caused depression. Potentiation was associated with an increased expression of surface alpha7 subunits and was not accompanied by detectable changes in receptor open probability, implying that the increased function results from an increased number of alpha7 nAChRs. Soluble N-ethylmaleimide-sensitive factor attachment protein receptor-mediated exocytosis was shown to be a plausible mechanism for the rapid delivery of additional alpha7 nAChRs to the plasma membrane. Direct phosphorylation/dephosphorylation of alpha7 subunits was unlikely because mutation of all three cytoplasmic tyrosine residues did not prevent the genistein-mediated facilitation. Overall, these data are consistent with the hypothesis that the number of functional cell surface alpha7 nAChRs is controlled indirectly via processes involving tyrosine phosphorylation.
Figures




Similar articles
-
Alpha7 neuronal nicotinic acetylcholine receptors are negatively regulated by tyrosine phosphorylation and Src-family kinases.J Neurosci. 2005 Oct 26;25(43):9836-49. doi: 10.1523/JNEUROSCI.3497-05.2005. J Neurosci. 2005. PMID: 16251431 Free PMC article.
-
A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization.J Neurosci. 2005 Apr 27;25(17):4396-405. doi: 10.1523/JNEUROSCI.5269-04.2005. J Neurosci. 2005. PMID: 15858066 Free PMC article.
-
Unexpected sensitivity of the human alpha7 neuronal nicotinic acetylcholine receptor to aminoglycosides.Neuroreport. 2006 Jan 23;17(1):65-70. doi: 10.1097/01.wnr.0000192736.85287.2f. Neuroreport. 2006. PMID: 16361952
-
Distinct profiles of alpha7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes.Mol Pharmacol. 2007 Sep;72(3):715-24. doi: 10.1124/mol.107.035410. Epub 2007 Jun 12. Mol Pharmacol. 2007. PMID: 17565004
-
Rat nicotinic ACh receptor alpha7 and beta2 subunits co-assemble to form functional heteromeric nicotinic receptor channels.J Physiol. 2002 Apr 15;540(Pt 2):425-34. doi: 10.1113/jphysiol.2001.013847. J Physiol. 2002. PMID: 11956333 Free PMC article.
Cited by
-
Resistance to Inhibitors of Cholinesterase 3 (Ric-3) Expression Promotes Selective Protein Associations with the Human α7-Nicotinic Acetylcholine Receptor Interactome.PLoS One. 2015 Aug 10;10(8):e0134409. doi: 10.1371/journal.pone.0134409. eCollection 2015. PLoS One. 2015. PMID: 26258666 Free PMC article.
-
Targeting Neuronal Alpha7 Nicotinic Acetylcholine Receptor Upregulation in Age-Related Neurological Disorders.Cell Mol Neurobiol. 2025 Jul 16;45(1):70. doi: 10.1007/s10571-025-01586-6. Cell Mol Neurobiol. 2025. PMID: 40668334 Free PMC article. Review.
-
Tyrosine phosphorylation differentially fine-tunes ionotropic and metabotropic responses of human α7 nicotinic acetylcholine receptor.Cell Mol Life Sci. 2021 Jul;78(13):5381-5395. doi: 10.1007/s00018-021-03853-3. Epub 2021 May 24. Cell Mol Life Sci. 2021. PMID: 34028590 Free PMC article.
-
T-cell receptor activation decreases excitability of cortical interneurons by inhibiting α7 nicotinic receptors.J Neurosci. 2014 Jan 1;34(1):22-35. doi: 10.1523/JNEUROSCI.2093-13.2014. J Neurosci. 2014. PMID: 24381265 Free PMC article.
-
Nicotine activates and up-regulates nicotinic acetylcholine receptors in bronchial epithelial cells.Am J Respir Cell Mol Biol. 2009 Jul;41(1):93-9. doi: 10.1165/rcmb.2008-0352OC. Epub 2008 Dec 18. Am J Respir Cell Mol Biol. 2009. PMID: 19097990 Free PMC article.
References
-
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y (1987) Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262: 5592-5595. - PubMed
-
- Alkondon M, Reinhardt S, Lobron C, Hermsen B, Maelicke A, Albuquerque EX (1994) Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. II. The rundown and inward rectification of agonist-elicited whole-cell currents and identification of receptor subunits by in situ hybridization. J Pharmacol Exp Ther 271: 494-506. - PubMed
-
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX (1997a) Neuronal nicotinic acetylcholine receptor activation modulates γ-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther 283: 1396-1411. - PubMed
-
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX (1997b) Choline is a selective agonist of α7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci 9: 2734-2742. - PubMed
-
- Amin J, Weiss DS (1994) Homomeric ρ1 GABA channels: activation properties and domains. Receptors Channels 2: 227-236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
