Site-specific incorporation of N-(deoxyguanosin-8-yl)-2-acetylaminofluorene (dG-AAF) into oligonucleotides using modified 'ultra-mild' DNA synthesis
- PMID: 15814813
- PMCID: PMC1074722
- DOI: 10.1093/nar/gki335
Site-specific incorporation of N-(deoxyguanosin-8-yl)-2-acetylaminofluorene (dG-AAF) into oligonucleotides using modified 'ultra-mild' DNA synthesis
Abstract
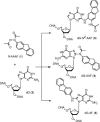
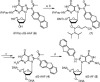
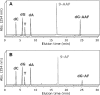
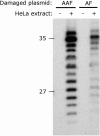
Aromatic amino and nitro compounds are potent carcinogens found in the environment that exert their toxic effects by reacting with DNA following metabolic activation. One important adduct is N-(deoxyguanosin-8-yl)-2-acetylaminofluorene (dG-AAF), which has been extensively used in studies of the mechanisms of DNA repair and mutagenesis. Despite the importance of dG-AAF adducts in DNA, an efficient method for its incorporation into DNA using solid-phase synthesis is still missing. We report the development of a modified 'ultra-mild' DNA synthesis protocol that allows the incorporation of dG-AAF into oligonucleotides of any length accessible by solid-phase DNA synthesis with high efficiency and independent of sequence context. Key to this endeavor was the development of improved deprotection conditions (10% diisopropylamine in methanol supplemented with 0.25 M of beta-mercaptoethanol) designed to remove protecting groups of commercially available 'ultra-mild' phosphoramidite building blocks without compromising the integrity of the exquisitely base-labile acetyl group at N8 of dG-AAF. We demonstrate the suitability of these oligonucleotides in the nucleotide excision repair reaction. Our synthetic approach should facilitate comprehensive studies of the mechanisms of repair and mutagenesis induced by dG-AAF adducts in DNA and should be of general use for the incorporation of base-labile functionalities into DNA.
Figures
References
-
- Beije B., Moller L. 2-Nitrofluorene and related compounds: prevalence and biological effects. Mutat. Res. 1988;196:177–209. - PubMed
-
- Heflich R.H., Neft R.E. Genetic toxicity of 2-acetylaminofluorene, 2-aminofluorene and some of their metabolites and model metabolites. Mutat. Res. 1994;318:73–174. - PubMed
-
- Schut H.A.J., Snyderwine E.G. DNA adducts of heterocyclic amine food mutagens: implications for mutagenesis and carcinogenesis. Carcinogenesis. 1999;20:353–368. - PubMed
-
- Kriek E., Miller J.A., Juhl U., Miller E.C. 8-(N-2-Fluorenylacetamido)guanosine, an arylamidation reaction product of guanosine and the carcinogen N-acetoxy-N-2-fluorenylacetamide in neutral solution. Biochemistry. 1967;6:177–182. - PubMed
-
- Pierce J.R., Case R., Tang M.S. Recognition and repair of 2-aminofluorene- and 2-(acetylamino)fluorene-DNA adducts by UVRABC nuclease. Biochemistry. 1989;28:5821–5826. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

