Nuclear targeting of adenovirus type 2 requires CRM1-mediated nuclear export
- PMID: 15814838
- PMCID: PMC1142442
- DOI: 10.1091/mbc.e05-02-0121
Nuclear targeting of adenovirus type 2 requires CRM1-mediated nuclear export
Abstract
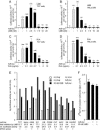
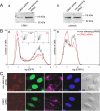
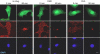
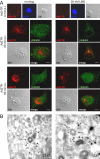
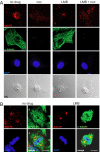
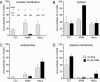
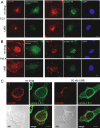
Incoming adenovirus type 2 (Ad2) and Ad5 shuttle bidirectionally along microtubules, biased to the microtubule-organizing center by the dynein/dynactin motor complex. It is unknown how the particles reach the nuclear pore complex, where capsids disassemble and viral DNA enters the nucleus. Here, we identified a novel link between nuclear export and microtubule-mediated transport. Two distinct inhibitors of the nuclear export factor CRM1, leptomycin B (LMB) and ratjadone A (RJA) or CRM1-siRNAs blocked adenovirus infection, arrested cytoplasmic transport of viral particles at the microtubule-organizing center or in the cytoplasm and prevented capsid disassembly and nuclear import of the viral genome. In mitotic cells where CRM1 is in the cytoplasm, adenovirus particles were not associated with microtubules but upon LMB treatment, they enriched at the spindle poles implying that CRM1 inhibited microtubule association of adenovirus. We propose that CRM1, a nuclear factor exported by CRM1 or a protein complex containing CRM1 is part of a sensor mechanism triggering the unloading of the incoming adenovirus particles from microtubules proximal to the nucleus of interphase cells.
Figures
Similar articles
-
CRM1 Promotes Capsid Disassembly and Nuclear Envelope Translocation of Adenovirus Independently of Its Export Function.J Virol. 2022 Feb 9;96(3):e0127321. doi: 10.1128/JVI.01273-21. Epub 2021 Nov 10. J Virol. 2022. PMID: 34757845 Free PMC article.
-
The nuclear export factor CRM1 controls juxta-nuclear microtubule-dependent virus transport.J Cell Sci. 2017 Jul 1;130(13):2185-2195. doi: 10.1242/jcs.203794. Epub 2017 May 17. J Cell Sci. 2017. PMID: 28515232
-
Ratjadone and leptomycin B block CRM1-dependent nuclear export by identical mechanisms.FEBS Lett. 2004 Oct 8;576(1-2):27-30. doi: 10.1016/j.febslet.2004.08.056. FEBS Lett. 2004. PMID: 15474004
-
Nuclear export of proteins and drug resistance in cancer.Biochem Pharmacol. 2012 Apr 15;83(8):1021-32. doi: 10.1016/j.bcp.2011.12.016. Epub 2011 Dec 20. Biochem Pharmacol. 2012. PMID: 22209898 Free PMC article. Review.
-
Inhibition of CRM1-dependent nuclear export sensitizes malignant cells to cytotoxic and targeted agents.Semin Cancer Biol. 2014 Aug;27:62-73. doi: 10.1016/j.semcancer.2014.03.001. Epub 2014 Mar 12. Semin Cancer Biol. 2014. PMID: 24631834 Free PMC article. Review.
Cited by
-
Nup358 and Transportin 1 Cooperate in Adenoviral Genome Import.J Virol. 2020 May 4;94(10):e00164-20. doi: 10.1128/JVI.00164-20. Print 2020 May 4. J Virol. 2020. PMID: 32161167 Free PMC article.
-
Virus trafficking - learning from single-virus tracking.Nat Rev Microbiol. 2007 Mar;5(3):197-208. doi: 10.1038/nrmicro1615. Nat Rev Microbiol. 2007. PMID: 17304249 Free PMC article. Review.
-
Viral Subversion of the Chromosome Region Maintenance 1 Export Pathway and Its Consequences for the Cell Host.Viruses. 2023 Nov 6;15(11):2218. doi: 10.3390/v15112218. Viruses. 2023. PMID: 38005895 Free PMC article. Review.
-
CRM1 Promotes Capsid Disassembly and Nuclear Envelope Translocation of Adenovirus Independently of Its Export Function.J Virol. 2022 Feb 9;96(3):e0127321. doi: 10.1128/JVI.01273-21. Epub 2021 Nov 10. J Virol. 2022. PMID: 34757845 Free PMC article.
-
CRM1-mediated nuclear export of dengue virus RNA polymerase NS5 modulates interleukin-8 induction and virus production.J Biol Chem. 2009 Jun 5;284(23):15589-97. doi: 10.1074/jbc.M808271200. Epub 2009 Mar 18. J Biol Chem. 2009. PMID: 19297323 Free PMC article.
References
-
- Alonso, C., Miskin, J., Hernaez, B., Fernandez-Zapatero, P., Soto, L., Canto, C., Rodriguez-Crespo, I., Dixon, L., and Escribano, J. M. (2001). African swine fever virus protein p54 interacts with the microtubular motor complex through direct binding to light-chain dynein. J. Virol. 75, 9819-9827. - PMC - PubMed
-
- Bednenko, J., Cingolani, G., and Gerace, L. (2003). Nucleocytoplasmic transport: navigating the channel. Traffic 4, 127-135. - PubMed
-
- Bienz, M. (2002). The subcellular destinations of APC proteins. Nat. Rev. Mol. Cell. Biol. 3, 328-338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

