Critical amino acid residues involved in the electrogenic sodium-bicarbonate cotransporter kNBC1-mediated transport
- PMID: 15817634
- PMCID: PMC1464572
- DOI: 10.1113/jphysiol.2005.084988
Critical amino acid residues involved in the electrogenic sodium-bicarbonate cotransporter kNBC1-mediated transport
Abstract
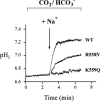
We have previously reported a topological model of the electrogenic Na(+)-HCO(3)(-) cotransporter (NBC1) in which the cotransporter spans the plasma membrane 10 times with N- and C-termini localized intracellularly. An analysis of conserved amino acid residues among members of the SLC4 superfamily in both the transmembrane segments (TMs) and intracellular/extracellular loops (ILs/ELs) provided the basis for the mutagenesis approach taken in the present study to determine amino acids involved in NBC1-mediated ion transport. Using large-scale mutagenesis, acidic and basic amino acids putatively involved in ion transport mediated by the predominant variant of NBC1 expressed in the kidney (kNBC1) were mutated to neutral and/or oppositely charged amino acids. All mutant kNBC1 cotransporters were expressed in HEK-293T cells and the Na(+)-dependent base flux of the mutants was determined using intracellular pH measurements with 2',7'-bis-(carboxyethyl)-5(6)-carboxyfluorescein (BCECF). Critical glutamate, aspartate, lysine, arginine and histidine residues in ILs/ELs and TMs were detected that were essential for kNBC1-mediated Na(+)-dependent base transport. In addition, critical phenylalanine, serine, tyrosine, threonine and alanine residues in TMs and ILs/ELs were detected. Furthermore, several amino acid residues in ILs/ELs and TMs were shown to be essential for membrane targeting. The data demonstrate asymmetry of distribution of kNBC1 charged amino acids involved in ion recognition in putative outward-facing and inward-facing conformations. A model summarizing key amino acid residues involved in kNBC1-mediated ion transport is presented.
Figures





References
-
- Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem. 1998;273:17689–17695. - PubMed
-
- Abuladze N, Song M, Pushkin A, Newman D, Lee I, Nicholas S, Kurtz I. Structural organization of the human NBC1 gene: kNBC1 is transcribed from an alternative promoter in intron 3. Gene. 2000;251:109–122. - PubMed
-
- Alper SL. Genetic deseases of acid-base transporters. Annu Rev Physiol. 2002;64:899–923. - PubMed
-
- Bok D, Schibler MJ, Pushkin A, Sassani P, Abuladze N, Naser Z, Kurtz I. Immunolocalization of electrogenic sodium-bicarbonate cotransporters pNBC1 and kNBC1 in the rat eye. Am J Physiol Renal Physiol. 2001;281:F920–F935. - PubMed
-
- Burnham CE, Amlal H, Wang Z, Shull GE, Soleimani M. Cloning and functional expression of a human kidney Na+:HCO3− cotransporter. J Biol Chem. 1997;272:19111–19114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

