Identification of a stem cell candidate in the normal human prostate gland
- PMID: 15819412
- PMCID: PMC2730953
- DOI: 10.1016/j.ejcb.2004.12.019
Identification of a stem cell candidate in the normal human prostate gland
Abstract
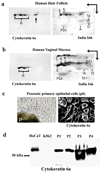
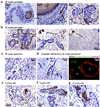
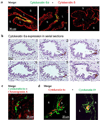
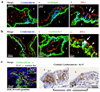
Stem cells of the human prostate gland have not yet been identified utilizing a structural biomarker. We have discovered a new prostatic epithelial cell phenotype-expressing cytokeratin 6a (Ck6a+ cells). The Ck6a+ cells are present within a specialized niche in the basal cell compartment in fetal, juvenile and adult prostate tissue, and within the stem cell-enriched urogenital sinus. In adult normal prostate tissue, the average abundance of Ck6a+ cells was 4.9%. With proliferative stimuli in the prostate organ culture model, in which the epithelial-stromal interaction was maintained, a remarkable increase of Ck6a expression was noticed to up to 64.9%. The difference in cytokeratin 6a expression between the normal adult prostate and the prostate organ culture model was statistically significant (p<0.0001). Within the prostate organ culture model the increase of cytokeratin 6a-expressing cells significantly correlated with increased proliferation index (r = 0.7616, p = 0.0467). The Ck6a+ cells were capable of differentiation as indicated by their expression of luminal cell markers such as ZO-1 and prostate specific antigen (PSA). Our data indicate that Ck6a+ cells represent a prostatic epithelial stem cell candidate possessing high potential for proliferation and differentiation. Since the development of benign prostatic hyperplasia and prostate carcinogenesis are disorders of proliferation and differentiation, the Ck6a+ cells may represent a major element in the development of these diseases.
Figures
Similar articles
-
Epithelial development in the rat ventral prostate, anterior prostate and seminal vesicle.Acta Anat (Basel). 1996;155(2):81-93. doi: 10.1159/000147793. Acta Anat (Basel). 1996. PMID: 8828706
-
Cell differentiation lineage in the prostate.Differentiation. 2001 Oct;68(4-5):270-9. doi: 10.1046/j.1432-0436.2001.680414.x. Differentiation. 2001. PMID: 11776479
-
Differential expression of specific cytokeratin polypeptides in the basal and luminal epithelia of the human prostate.Prostate. 1991;18(4):303-14. doi: 10.1002/pros.2990180404. Prostate. 1991. PMID: 1711687
-
Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model.Prostate. 1996 Feb;28(2):98-106. doi: 10.1002/(SICI)1097-0045(199602)28:2<98::AID-PROS4>3.0.CO;2-J. Prostate. 1996. PMID: 8604398 Review.
-
Stem cell differentiation within the human prostate epithelium: implications for prostate carcinogenesis.BJU Int. 2001 Sep;88 Suppl 2:35-42; discussion 49-50. doi: 10.1046/j.1464-410x.2001.00117.x. BJU Int. 2001. PMID: 11589668 Review. No abstract available.
Cited by
-
Self-renewal and differentiation of rat epididymal basal cells using a novel in vitro organoid model†.Biol Reprod. 2021 Oct 11;105(4):987-1001. doi: 10.1093/biolre/ioab113. Biol Reprod. 2021. PMID: 34104939 Free PMC article.
-
Vitamin D sufficiency enhances differentiation of patient-derived prostate epithelial organoids.iScience. 2021 Jan 5;24(1):101974. doi: 10.1016/j.isci.2020.101974. eCollection 2021 Jan 22. iScience. 2021. PMID: 33458620 Free PMC article.
-
Stromal upregulation of lateral epithelial adhesions: gene expression analysis of signalling pathways in prostate epithelium.J Biomed Sci. 2011 Jun 22;18(1):45. doi: 10.1186/1423-0127-18-45. J Biomed Sci. 2011. PMID: 21696611 Free PMC article.
-
Progenitor cells are responsible for formation primary epithelial cultures in the prostate epithelial model.Int Urol Nephrol. 2007;39(3):851-7. doi: 10.1007/s11255-006-9105-6. Epub 2007 Feb 22. Int Urol Nephrol. 2007. PMID: 17318344
-
Characterization of Laminin Binding Integrin Internalization in Prostate Cancer Cells.J Cell Biochem. 2017 May;118(5):1038-1049. doi: 10.1002/jcb.25673. Epub 2017 Jan 5. J Cell Biochem. 2017. PMID: 27509031 Free PMC article.
References
-
- Achtstätter T, Hatzfeld M, Quinlan R, Parmelee D, Franke WW. Separation of cytokeratin polypeptides by gel electrophoretic and chromatographic techniques and their identification by immunoblotting. Methods Enzymol. 1986;134:355–371. - PubMed
-
- Barnett D, Huang H, Wu X, Laciak R, Shapir E, Bushman W. The human prostate expresses sonic hedgehog during fetal development. J. Urol. 2002;168:2206–2210. - PubMed
-
- Bierie B, Nozawa M, Renou JP, Shillingford JM, Morgan F, Oka T, Taketo MM, Cardiff RD, Miyoshi K, Wagner KU, Robinson GW, Hennighausen L. Activation of beta-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene. 2003;22:3875–3887. - PubMed
-
- Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 1999;116:7–14. - PubMed
-
- Bonkhoff H, Remberger K. Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model. Prostate. 1996;28:98–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

