Epicutaneous immunization induces alphabeta T-cell receptor CD4 CD8 double-positive non-specific suppressor T cells that inhibit contact sensitivity via transforming growth factor-beta
- PMID: 15819696
- PMCID: PMC1782121
- DOI: 10.1111/j.1365-2567.2005.02127.x
Epicutaneous immunization induces alphabeta T-cell receptor CD4 CD8 double-positive non-specific suppressor T cells that inhibit contact sensitivity via transforming growth factor-beta
Abstract
Since it was previously shown that protein antigens applied epicutaneously in mice induce allergic dermatitis mediated by production of T helper 2 (Th2) cytokines we postulated that this might induce suppression of Th1 immunity. Here we show that epicutaneous immunization of normal mice with a different protein antigen applied on the skin in the form of a patch induces a state of subsequent antigen-non-specific unresponsiveness caused by suppressor T cells (Ts) that inhibit sensitization and elicitation of effector T-cell responses. Suppression is transferable in vivo by alphabeta-T-cell receptor CD4(+) CD8(+) double positive lymphocytes harvested from lymphoid organs of skin patched animals and are not major histocompatibility complex-restricted nor antigen specific. Both CD25(+) and CD25(-) CD4(+) CD8(+) T cells are able to suppress adoptive transfer of Th1 effector cells mediating cutaneous contact sensitivity. In vivo treatment with monoclonal antibodies showed that the cytokines interleukin (IL)-4, IL-10 and transforming growth factor-beta (TGF-beta) are involved in the induction of the Ts cells. Additionally, using IL-10(-/-) mice we found that IL-10 is involved in skin induced tolerance. Further in vitro experiments showed that lymph node cells of skin tolerized mice non-specifically suppress [(3)H]thymidine incorporation by antigen-stimulated immune cells and this effect can be abolished by adding anti-TGF-beta, but not anti-IL-4 nor anti-IL-10 antibodies. These studies indicate the crucial role of TGF-beta in skin induced tolerance due to non-antigen-specific Ts cells and also show that IL-4, IL-10 and TGF-beta play an important role in the induction of epicutaneously induced Ts cell suppression.
Figures
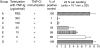





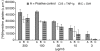

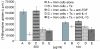
References
-
- Weiner HL, Friedman A, Miller A, et al. Oral tolerance: Immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–37. 10.1146/annurev.iy.12.040194.004113. - DOI - PubMed
-
- Xiao B-G, Link H. Mucosal tolerance: a two-edged sword to prevent and treat autoimmune diseases. Clin Immunol Immunopathol. 1997;85:119–28. 10.1006/clin.1997.4432. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

