Abacavir, efavirenz, didanosine, with or without hydroxyurea, in HIV-infected adults failing initial nucleoside/protease inhibitor-containing regimens
- PMID: 15819974
- PMCID: PMC1090576
- DOI: 10.1186/1471-2334-5-23
Abacavir, efavirenz, didanosine, with or without hydroxyurea, in HIV-infected adults failing initial nucleoside/protease inhibitor-containing regimens
Abstract
Background: Hydroxyurea (HU) is an immunomodulatory agent that has been documented to enhance the antiretroviral activity of nucleoside reverse transcriptase inhibitors, such as abacavir (ABC) and didanosine (ddI), and would be expected to improve virologic efficacy.
Methods: A 48-week, phase IV, multicenter, open-label, proof-of-concept clinical trial was conducted to evaluate second-line, protease inhibitor (PI)-sparing therapy with ABC/efavirenz (EFV)/ddI plus HU or without HU in HIV-infected subjects failing to achieve HIV-1 RNA < or = 400 copies/mL after > or = 16 weeks of treatment with lamivudine/zidovudine or lamivudine/stavudine, plus 1 or 2 PIs. Subjects were assigned to ABC (300 mg twice daily)/ EFV (600 mg once daily)/ ddI (400 mg once daily) plus HU (500 mg twice daily) (n = 30) or this regimen without HU (n = 24).
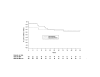
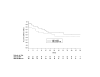
Results: Baseline mean HIV-1 RNA was 3.86 log10 copies/mL and CD4+ cell count was 345 cells/mm3. A similar percentage of subjects in the non-HU arm (58%) and HU arm (53%) completed the study. Intent-to-treat: missing = failure analysis showed no differences in proportions of subjects in the non-HU and HU arms achieving undetectable plasma HIV-1 RNA levels at week 24 (< 400 copies/mL: 58% [14/24] vs 57% [17/30], P = 0.899; < 50 copies/mL (50% [12/24] vs 47% [14/30], P = 0.780). Median change from baseline in CD4+ cell count in the non-HU and HU arms at week 48 was +114 cells/mm3 and -63 cells/mm3 (P = 0.007), respectively. Both regimens were generally well tolerated, although more subjects in the HU arm withdrew prematurely from the study due to adverse events (23% vs 4%). Four cases of possible ABC-related hypersensitivity were observed.
Conclusion: ABC/EFV/ddI was an effective and well-tolerated second-line regimen for nucleoside/PI-experienced HIV-infected subjects. The addition of HU blunted the CD4+ cell response, did not appear to enhance antiviral activity, and resulted in more treatment-limiting adverse events.
Figures



Similar articles
-
The HYDILE trial: efficacy and tolerance of a quadruple combination of reverse transcriptase inhibitors versus the same regimen plus hydroxyurea or hydroxyurea and interleukin-2 in HIV-infected patients failing protease inhibitor-based combinations.HIV Clin Trials. 2002 Jul-Aug;3(4):263-71. doi: 10.1310/X6B5-9K42-E25N-F680. HIV Clin Trials. 2002. PMID: 12187499 Clinical Trial.
-
Pilot study of once-daily simplification therapy with abacavir/lamivudine/zidovudine and efavirenz for treatment of HIV-1 infection.HIV Clin Trials. 2006 Sep-Oct;7(5):229-36. doi: 10.1310/hct0705-229. HIV Clin Trials. 2006. PMID: 17162316 Clinical Trial.
-
Compact quadruple therapy with the lamivudine/zidovudine combination tablet plus abacavir and efavirenz, followed by the lamivudine/zidovudine/abacavir triple nucleoside tablet plus efavirenz in treatment-naïve HIV-infected adults.HIV Clin Trials. 2003 Jul-Aug;4(4):231-43. doi: 10.1310/MM9W-BAU0-BT6Q-401B. HIV Clin Trials. 2003. PMID: 12916008 Clinical Trial.
-
Systematic review of combination antiretroviral therapy with didanosine plus hydroxyurea: a partial solution to Africa's HIV/AIDS problem?Int J Infect Dis. 2001;5(1):43-8. doi: 10.1016/s1201-9712(01)90048-7. Int J Infect Dis. 2001. PMID: 11285159
-
Efavirenz: a decade of clinical experience in the treatment of HIV.J Antimicrob Chemother. 2009 Nov;64(5):910-28. doi: 10.1093/jac/dkp334. Epub 2009 Sep 18. J Antimicrob Chemother. 2009. PMID: 19767318 Free PMC article. Review.
Cited by
-
Drug-Induced Acute Pancreatitis: An Evidence-Based Classification (Revised).Clin Transl Gastroenterol. 2023 Aug 1;14(8):e00621. doi: 10.14309/ctg.0000000000000621. Clin Transl Gastroenterol. 2023. PMID: 37440319 Free PMC article.
-
Systematic review: Hydroxyurea for the treatment of adults with sickle cell disease.Ann Intern Med. 2008 Jun 17;148(12):939-55. doi: 10.7326/0003-4819-148-12-200806170-00221. Epub 2008 May 5. Ann Intern Med. 2008. PMID: 18458272 Free PMC article.
References
-
- Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, Richman DD, Valentine FT, Jonas L, Meibohm A, Emini EA, Chodakewitz JA, Deutsch P, Holder D, Schleif WA, Condra JH. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. - DOI - PubMed
-
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, Eron JJ, Feinberg JE, Balfour HH, Deyton LR, Chodakewitz JA, Fischl MA, Phair JP, Pedneault L, Nguyen B-Y, Cook JC, for The AIDS Clinical Trials Group 320 Study Team A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. - DOI - PubMed
-
- Opravil M, Hirschel B, Lazzarin A, Furrer H, Chave J-P, Yerly S, Bisset LR, Fischer M, Vernazza P, Bernasconi E, Battegay M, Ledergerber B, Günthard H, Howe C, Weber R, Perrin L, for the Swiss HIV Cohort Study A randomized trial of simplified mantenance therapy with abacavir, lamivudine, and zidovudine in human immunodeficiency virus infection. J Infect Dis. 2002;185:1251–1260. doi: 10.1086/340312. - DOI - PubMed
-
- Katlama C, Fenske S, Gazzard B, Lazzarin A, Clumeck N, Mallolas J, Lafeuillade A, Mamet J-P, Beauvais L, on behalf of the AZL30002 European study team TRIZAL study: switching from successful HAART to Trizivir™ (abacavir-lamivudine-zidovudine combination tablet): 48 weeks efficacy, safety and adherence results. HIV Med. 2003;4:79–86. doi: 10.1046/j.1468-1293.2003.00139.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

