Deficiency in type 1 insulin-like growth factor receptor in mice protects against oxygen-induced lung injury
- PMID: 15819984
- PMCID: PMC1084363
- DOI: 10.1186/1465-9921-6-31
Deficiency in type 1 insulin-like growth factor receptor in mice protects against oxygen-induced lung injury
Abstract
Background: Cellular responses to aging and oxidative stress are regulated by type 1 insulin-like growth factor receptor (IGF-1R). Oxidant injury, which is implicated in the pathophysiology of a number of respiratory diseases, acutely upregulates IGF-1R expression in the lung. This led us to suspect that reduction of IGF-1R levels in lung tissue could prevent deleterious effects of oxygen exposure.
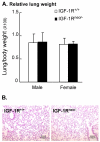
Methods: Since IGF-1R null mutant mice die at birth from respiratory failure, we generated compound heterozygous mice harboring a hypomorphic (Igf-1rneo) and a knockout (Igf-1r-) receptor allele. These IGF-1Rneo/- mice, strongly deficient in IGF-1R, were subjected to hyperoxia and analyzed for survival time, ventilatory control, pulmonary histopathology, morphometry, lung edema and vascular permeability.
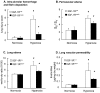
Results: Strikingly, after 72 h of exposure to 90% O2, IGF-1Rneo/- mice had a significantly better survival rate during recovery than IGF-1R+/+ mice (77% versus 53%, P < 0.05). The pulmonary injury was consistently, and significantly, milder in IGF-1Rneo/- mice which developed conspicuously less edema and vascular extravasation than controls. Also, hyperoxia-induced abnormal pattern of breathing which precipitated respiratory failure was elicited less frequently in the IGF-1Rneo/- mice.
Conclusion: Together, these data demonstrate that a decrease in IGF-1R signaling in mice protects against oxidant-induced lung injury.
Figures


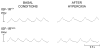

References
-
- Crapo JD, Barry BE, Foscue HA, Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980;122:123–143. - PubMed
-
- Barazzone C, Horowitz S, Donati YR, Rodriguez I, Piguet PF. Oxygen toxicity in mouse lung: pathways to cell death. Am J Respir Cell Mol Biol. 1998;19:573–581. - PubMed
-
- Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol. 1998;275:L110–7. - PubMed
-
- Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med. 1999;160:1333–1346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

