Effective antiprotease-antibiotic treatment of experimental anthrax
- PMID: 15819985
- PMCID: PMC1090577
- DOI: 10.1186/1471-2334-5-25
Effective antiprotease-antibiotic treatment of experimental anthrax
Abstract
Background: Inhalation anthrax is characterized by a systemic spread of the challenge agent, Bacillus anthracis. It causes severe damage, including multiple hemorrhagic lesions, to host tissues and organs. It is widely believed that anthrax lethal toxin secreted by proliferating bacteria is a major cause of death, however, the pathology of intoxication in experimental animals is drastically different from that found during the infectious process. In order to close a gap between our understanding of anthrax molecular pathology and the most prominent clinical features of the infectious process we undertook bioinformatic and experimental analyses of potential proteolytic virulence factors of B. anthracis distinct from lethal toxin.
Methods: Secreted proteins (other than lethal and edema toxins) produced by B. anthracis were tested for tissue-damaging activity and toxicity in mice. Chemical protease inhibitors and rabbit immune sera raised against B. anthracis proteases were used to treat mice challenged with B. anthracis (Sterne) spores.
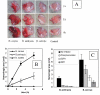
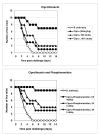
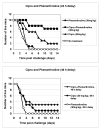
Results: B. anthracis strain delta Ames (pXO1-, pXO2-) producing no lethal and edema toxins secrets a number of metalloprotease virulence factors upon cultivation under aerobic conditions, including those with hemorrhagic, caseinolytic and collagenolytic activities, belonging to M4 and M9 thermolysin and bacterial collagenase families, respectively. These factors are directly toxic to DBA/2 mice upon intratracheal administration at 0.5 mg/kg and higher doses. Chemical protease inhibitors (phosphoramidon and 1, 10-phenanthroline), as well as immune sera against M4 and M9 proteases of B. anthracis, were used to treat mice challenged with B. anthracis (Sterne) spores. These substances demonstrate a substantial protective efficacy in combination with ciprofloxacin therapy initiated as late as 48 h post spore challenge, compared to the antibiotic alone.
Conclusion: Secreted proteolytic enzymes are important pathogenic factors of B. anthrasis, which can be considered as effective therapeutic targets in the development of anthrax treatment and prophylactic approaches complementing anti-lethal toxin therapy.
Figures


References
-
- Inglesby TV, O'Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Gerberding J, Hauer J, Hughes J, McDade J, Osterholm MT, Parker G, Perl TM, Russell PK, Tonat K. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA. 2002;287:2236–2252. doi: 10.1001/jama.287.17.2236. - DOI - PubMed
-
- Cui X, Moayeri M, Li Y, Li X, Haley M, Fitz Y, Correa-Araujo R, Banks SM, Leppla SH, Eichacker PQ. Lethality during continuous anthrax lethal toxin infusion is associated with circulatory shock but not inflammatory cytokine or nitric oxide release in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R699–709. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

