Hsp27 and axonal growth in adult sensory neurons in vitro
- PMID: 15819993
- PMCID: PMC1087488
- DOI: 10.1186/1471-2202-6-24
Hsp27 and axonal growth in adult sensory neurons in vitro
Abstract
Background: Neurite growth can be elicited by growth factors and interactions with extracellular matrix molecules like laminin. Among the targets of the signalling pathways activated by these stimuli are cytoskeletal elements, such as actin, tubulin and neurofilaments. The cytoskeleton can also be modulated by other proteins, such as the small heat shock protein Hsp27. Hsp27 interacts with actin and tubulin in non-neuronal cells and while it has been suggested to play a role in the response of some neurons to injury, there have been no direct studies of its contribution to axonal regeneration.
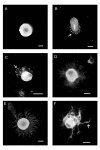
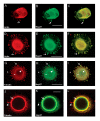
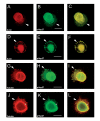
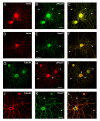
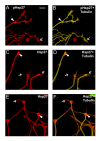
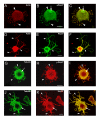
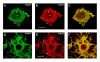
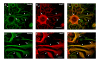
Results: We have investigated neurite initiation and process extension using cultures of adult dorsal root ganglion (DRG) sensory neurons and a laminin stimulation paradigm. Employing confocal microscopy and biochemical analyses we have examined localization of Hsp27 at early and later stages of neurite growth. Our results show that Hsp27 is colocalized with actin and tubulin in lamellopodia, filopodia, focal contacts and mature neurites and growth cones. Disruption of the actin cytoskeleton with cytochalasin D results in aberrant neurite initiation and extension, effects which may be attributable to alterations in actin polymerization states. Inhibition of Hsp27 phosphorylation in our cultures results in an atypical growth pattern that may be attributable to an effect of pHsp27 on the stability of the actin cytoskeleton.
Conclusion: We observed colocalization of the phosphorylated and non-phosphorylated forms of Hsp27 with actin and tubulin in both very early and later stages of neurite growth from cultured adult DRG neurons. The colocalization of Hsp27 and pHsp27 with actin in lamellopodia and focal contacts at early stages of neurite growth, and in processes, branch points and growth cones at later stages, suggests that Hsp27 may play a role in neuritogenesis and subsequent neurite extension, and potentially in the patterning of this growth. Hsp27 has been reported to play a key role in modulating actin cytoskeletal dynamics as an actin-capping protein in non-neuronal cells. Our results suggest that this may also be the case in neurons and support a role for Hsp27 in neurite outgrowth via its phosphorylation state-dependent interactions with actin.
Figures

References
-
- Tucker BA, Rahimtula M, Mearow KM. Integrin activation and neurotrophin signaling cooperate to enhance neurite outgrowth in sensory neurons. J Comp Neurol. 2005;486 - PubMed
-
- Charette SJ, Landry J. The interaction of HSP27 with Daxx identifies a potential regulatory role of HSP27 in Fas-induced apoptosis. Ann N Y Acad Sci. 2000;926:126–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

