Neuroprotective activity of the mGluR5 antagonists MPEP and MTEP against acute excitotoxicity differs and does not reflect actions at mGluR5 receptors
- PMID: 15821750
- PMCID: PMC1576169
- DOI: 10.1038/sj.bjp.0706219
Neuroprotective activity of the mGluR5 antagonists MPEP and MTEP against acute excitotoxicity differs and does not reflect actions at mGluR5 receptors
Abstract
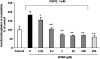
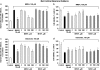
1 Neuroprotection has been reported after either activation or blockade of the group I metabotropic glutamate receptor subtype 5 (mGluR5). However, some recent evidence suggests that protection provided by mGluR5 antagonists may reflect their ability to inhibit N-methyl-D-aspartate (NMDA) receptor activity. 2 Here, in both rat and mouse cortical neurons, we compare the neuroprotective actions of two mGluR5 antagonists: 2-methyl-6-(phenylethynyl)-pyridine (MPEP), which has been commonly used and 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP), a more recently developed compound believed to have greater mGluR5 selectivity. We have previously shown that MPEP directly reduces single-channel NMDA receptor open time at the same concentrations (20 microM or greater) that show neuroprotection, whereas MPEP antagonizes mGluR5 agonist ((RS)-2-chloro-5-hydroxyphenylglycine (CHPG))-induced changes in inositol phosphates (IP) at concentrations as low as 0.2 microM. 3 In the present studies, MTEP significantly inhibited CHPG-mediated IP hydrolysis at concentrations as low as 0.02 microM. In contrast to MPEP, which significantly reduced glutamate- or NMDA-mediated cell death in primary rat neuronal cultures at a concentration of 20 microM, small neuroprotective effects were observed with MTEP only at a concentration of 200 microM. Neither MPEP- nor MTEP-mediated mGluR5 inhibition had any effect on etoposide-induced apoptotic cell death. In rat cortical neurons, the neuroprotective effects of MTEP at very high concentrations, like those of MPEP, reflect ability to directly reduce NMDA receptor peak and steady-state currents. 4 We also compared the effects of MPEP and MTEP in primary cortical neuronal cultures from parental and mGluR5 knockout mice. Both agents were neuroprotective, at high concentrations in normal as well as in the knockout cultures. In contrast to rat cortical neurons, neither MPEP nor MTEP appears to directly alter NMDA receptor activity. 5 Combined, these studies support the conclusion that MTEP has greater mGluR5 selectivity than MPEP, and that neuroprotection provided by either antagonist in neuronal cultures does not reflect inhibition of mGluR5 receptors.
Figures
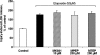



Similar articles
-
Selective mGluR5 antagonists MPEP and SIB-1893 decrease NMDA or glutamate-mediated neuronal toxicity through actions that reflect NMDA receptor antagonism.Br J Pharmacol. 2000 Dec;131(7):1429-37. doi: 10.1038/sj.bjp.0703715. Br J Pharmacol. 2000. PMID: 11090117 Free PMC article.
-
mGluR5 antagonists 2-methyl-6-(phenylethynyl)-pyridine and (E)-2-methyl-6-(2-phenylethenyl)-pyridine reduce traumatic neuronal injury in vitro and in vivo by antagonizing N-methyl-D-aspartate receptors.J Pharmacol Exp Ther. 2001 Jan;296(1):41-7. J Pharmacol Exp Ther. 2001. PMID: 11123360
-
Selective mGluR5 receptor antagonist or agonist provides neuroprotection in a rat model of focal cerebral ischemia.Brain Res. 2001 Dec 20;922(2):173-9. doi: 10.1016/s0006-8993(01)03062-1. Brain Res. 2001. PMID: 11743947
-
Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP.CNS Drug Rev. 2006 Summer;12(2):149-66. doi: 10.1111/j.1527-3458.2006.00149.x. CNS Drug Rev. 2006. PMID: 16958988 Free PMC article. Review.
-
The role of metabotropic glutamate receptor 5 in learning and memory processes.Drug News Perspect. 2005 Jul-Aug;18(6):353-61. doi: 10.1358/dnp.2005.18.6.927927. Drug News Perspect. 2005. PMID: 16247513 Review.
Cited by
-
Activation of mGluR5 Attenuates Microglial Activation and Neuronal Apoptosis in Early Brain Injury After Experimental Subarachnoid Hemorrhage in Rats.Neurochem Res. 2015 Jun;40(6):1121-32. doi: 10.1007/s11064-015-1572-7. Epub 2015 Apr 7. Neurochem Res. 2015. PMID: 25846008
-
Fragile X Syndrome and Alzheimer's Disease: Another story about APP and beta-amyloid.Curr Alzheimer Res. 2010 May;7(3):200-6. doi: 10.2174/156720510791050957. Curr Alzheimer Res. 2010. PMID: 20088809 Free PMC article. Review.
-
ITPR2 Mediated Calcium Homeostasis in Oligodendrocytes is Essential for Myelination and Involved in Depressive-Like Behavior in Adolescent Mice.Adv Sci (Weinh). 2024 May;11(20):e2306498. doi: 10.1002/advs.202306498. Epub 2024 Mar 13. Adv Sci (Weinh). 2024. PMID: 38476116 Free PMC article.
-
Effects of the mGluR5 antagonist MPEP on ethanol withdrawal induced anxiety-like syndrome in rats.Behav Brain Funct. 2013 Nov 26;9:43. doi: 10.1186/1744-9081-9-43. Behav Brain Funct. 2013. PMID: 24279870 Free PMC article.
-
Progress toward therapeutic potential for AFQ056 in Fragile X syndrome.J Exp Pharmacol. 2013 Jul 16;5:45-54. doi: 10.2147/JEP.S27044. eCollection 2013. J Exp Pharmacol. 2013. PMID: 27186135 Free PMC article. Review.
References
-
- AGRAWAL S.K., THERIAULT E., FEHLINGS M.G. Role of group I metabotropic glutamate receptors in traumatic spinal cord white matter injury. J. Neurotrauma. 1998;15:929–941. - PubMed
-
- ALLEN J.W., ELDADAH B.A., FADEN A.I. Beta-amyloid-induced apoptosis of cerebellar granule cells and cortical neurons: exacerbation by selective inhibition of group I metabotropic glutamate receptors. Neuropharmacology. 1999;38:1243–1252. - PubMed
-
- ALLEN J.W., KNOBLACH S.M., FADEN A.I. Activation of group I metabotropic glutamate receptors reduces neuronal apoptosis but increases necrotic cell death in vitro. Cell Death Differ. 2000;7:470–476. - PubMed
-
- ALLEN J.W., VICINI S., FADEN A.I. Exacerbation of neuronal cell death by activation of group I metabotropic glutamate receptors: role of NMDA receptors and arachidonic acid release. Exp. Neurol. 2001;169:449–460. - PubMed
-
- ANDERSON J.J., BRADBURY M.J., GIRACELLO D.R., CHAPMAN D.F., HOLTZ G., ROPPE J., KING C., COSFORD N.D., VARNEY M.A. In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]3-methoxy-5-(pyridin-2-ylethynyl)pyridine) Eur. J. Pharmacol. 2003;473:35–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

