Inhibition of a yeast LTR retrotransposon by human APOBEC3 cytidine deaminases
- PMID: 15823539
- PMCID: PMC2839876
- DOI: 10.1016/j.cub.2005.02.051
Inhibition of a yeast LTR retrotransposon by human APOBEC3 cytidine deaminases
Abstract
The mammalian APOBEC3 family of cytidine deaminases includes several members that possess potent antiretroviral activity. Human APOBEC3F and APOBEC3G are specifically incorporated into human immunodeficiency virus type 1 (HIV-1) progeny virions in the absence of virion infectivity factor (Vif), where they deaminate deoxycytidine to deoxyuridine on the minus strand of nascent reverse transcripts. Editing of the HIV-1 cDNA leads to its degradation or to G to A hypermutation of the integrated provirus. Here, we show that APOBEC3 proteins also restrict the activity of a distantly related long terminal repeat (LTR) retrotransposon. When expressed in the yeast Saccharomyces cerevisiae, human APOBEC3C, APOBEC3F, or APOBEC3G or mouse APOBEC3 potently inhibit replication of the Ty1 LTR retrotransposon. APOBEC3G interacts with Ty1 Gag and is packaged into Ty1 virus-like particles (VLPs) by a mechanism that closely resembles the one it uses to enter HIV-1 virions. Expression of APOBEC3G results in a reduced level of Ty1 cDNA integration and G to A editing of integrated Ty1 cDNA. Our findings indicate that APOBEC3G restricts Ty1 and HIV-1 by similar mechanisms and suggest that the APOBEC3 proteins target a substantially broader spectrum of retroelements than previously appreciated.
Figures
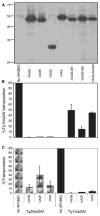



References
-
- Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. - PubMed
-
- Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. - PubMed
-
- Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, Watt IN, Neuberger MS, Malim MH. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–809. - PubMed
-
- Mariani R, Chen D, Schrofelbauer B, Navarro F, Konig R, Bollman B, Munk C, Nymark-McMahon H, Landau NR. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell. 2003;114:21–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

