Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma
- PMID: 15824083
- PMCID: PMC2213148
- DOI: 10.1084/jem.20041948
Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma
Abstract
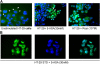
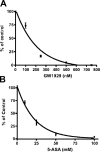
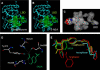
5-aminosalicylic acid (5-ASA) is an antiinflammatory drug widely used in the treatment of inflammatory bowel diseases. It is known to inhibit the production of cytokines and inflammatory mediators, but the mechanism underlying the intestinal effects of 5-ASA remains unknown. Based on the common activities of peroxisome proliferator-activated receptor-gamma (PPAR-gamma) ligands and 5-ASA, we hypothesized that this nuclear receptor mediates 5-ASA therapeutic action. To test this possibility, colitis was induced in heterozygous PPAR-gamma(+/-) mice and their wild-type littermates, which were then treated with 5-ASA. 5-ASA treatment had a beneficial effect on colitis only in wild-type and not in heterozygous mice. In epithelial cells, 5-ASA increased PPAR-gamma expression, promoted its translocation from the cytoplasm to the nucleus, and induced a modification of its conformation permitting the recruitment of coactivators and the activation of a peroxisome-proliferator response element-driven gene. Validation of these results was obtained with organ cultures of human colonic biopsies. These data identify PPAR-gamma as a target of 5-ASA underlying antiinflammatory effects in the colon.
Figures






References
-
- Svartz, N. 1941. Ett nytt sulfonamidpreparat? Forelopande meddelande [Swedish]. Nord. Med. 9:544.
-
- Azad Khan, A.K., J. Piris, and S.C. Truelove. 1977. An experiment to determine the active therapeutic moiety of sulphasalazine. Lancet. 2:892–895. - PubMed
-
- Brogden, R.N., and E.M. Sorkin. 1989. Mesalazine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in chronic inflammatory bowel disease. Drugs. 38:500–523. - PubMed
-
- Greenfield, S.M., N.A. Punchard, J.P. Teare, and R.P. Thompson. 1993. The mode of action of the aminosalicylates in inflammatory bowel disease. Aliment. Pharmacol. Ther. 7:369–383. - PubMed
-
- Zhou, S.Y., D. Fleisher, L.H. Pao, C. Li, B. Winward, and E.M. Zimmermann. 1999. Intestinal metabolism and transport of 5-aminosalicylate. Drug Metab. Dispos. 27:479–485. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

