CD44 is a physiological E-selectin ligand on neutrophils
- PMID: 15824084
- PMCID: PMC2213157
- DOI: 10.1084/jem.20042014
CD44 is a physiological E-selectin ligand on neutrophils
Abstract
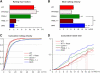
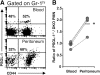
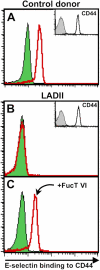
The selectin family of adhesion molecules and their glycoconjugated ligands are essential for blood polymorphonuclear neutrophil (PMN) extravasation into inflammatory and infectious sites. However, E-selectin ligands on PMNs are not well characterized. We show here that CD44 immunopurified from G-CSF-differentiated 32D cells or from peripheral blood PMNs binds specifically to E-selectin. In contrast, CD44 extracted from bone marrow stromal or brain endothelial cell lines does not interact with E-selectin, suggesting cell-specific posttranslational modifications of CD44. PMN-derived CD44 binding activity is mediated by sialylated, alpha(1,3) fucosylated, N-linked glycans. CD44 enables slow leukocyte rolling on E-selectin expressed on inflamed endothelium in vivo and cooperates with P-selectin glycoprotein ligand-1 to recruit neutrophils into thioglycollate-induced peritonitis and staphylococcal enterotoxin A-injected skin pouch. CD44 extracted from human PMNs also binds to E-selectin. Moreover, we demonstrate that CD44 is hypofucosylated in PMNs from a patient with leukocyte adhesion deficiency type II, suggesting that it contributes to the syndrome. These findings thus suggest broader roles for CD44 in the innate immune response and uncover a potential new target for diseases in which selectins play a prominent role.
Figures

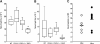
References
-
- Lowe, J.B. 2003. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr. Opin. Cell Biol. 15:531–538. - PubMed
-
- Ley, K. 2003. The role of selectins in inflammation and disease. Trends Mol. Med. 9:263–268. - PubMed
-
- Steegmaier, M., A. Levinovitz, S. Isenmann, E. Borges, M. Lenter, H.P. Kocher, B. Kleuser, and D. Vestweber. 1995. The E-selectin-ligand ESL-1 is a variant of a receptor for fibroblast growth factor. Nature. 373:615–620. - PubMed
-
- Jones, W.M., G.M. Watts, M.K. Robinson, D. Vestweber, and M.A. Jutila. 1997. Comparison of E-selectin-binding glycoprotein ligands on human lymphocytes, neutrophils, and bovine gamma delta T cells. J. Immunol. 159:3574–3583. - PubMed
-
- Montoya, M.C., K. Holtmann, K.R. Snapp, E. Borges, F. Sanchez-Madrid, F.W. Luscinskas, G. Kansas, D. Vestweber, and M.O. de Landazuri. 1999. Memory B lymphocytes from secondary lymphoid organs interact with E-selectin through a novel glycoprotein ligand. J. Clin. Invest. 103:1317–1327. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

