Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers
- PMID: 15824085
- PMCID: PMC2213151
- DOI: 10.1084/jem.20042028
Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers
Abstract
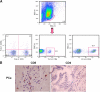
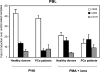
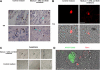
Immunotherapy may provide valid alternative therapy for patients with hormone-refractory metastatic prostate cancer. However, if the tumor environment exerts a suppressive action on antigen-specific tumor-infiltrating lymphocytes (TIL), immunotherapy will achieve little, if any, success. In this study, we analyzed the modulation of TIL responses by the tumor environment using collagen gel matrix-supported organ cultures of human prostate carcinomas. Our results indicate that human prostatic adenocarcinomas are infiltrated by terminally differentiated cytotoxic T lymphocytes that are, however, in an unresponsive status. We demonstrate the presence of high levels of nitrotyrosines in prostatic TIL, suggesting a local production of peroxynitrites. By inhibiting the activity of arginase and nitric oxide synthase, key enzymes of L-arginine metabolism that are highly expressed in malignant but not in normal prostates, reduced tyrosine nitration and restoration of TIL responsiveness to tumor were achieved. The metabolic control exerted by the tumor on TIL function was confirmed in a transgenic mouse prostate model, which exhibits similarities with human prostate cancer. These results identify a novel and dominant mechanism by which cancers induce immunosuppression in situ and suggest novel strategies for tumor immunotherapy.
Figures





References
-
- Finn, O.J. 2003. Cancer vaccines: between the idea and the reality. Nat. Rev. Immunol. 3:630–641. - PubMed
-
- Van Der Bruggen, P., Y. Zhang, P. Chaux, V. Stroobant, C. Panichelli, E.S. Schultz, J. Chapiro, B.J. Van Den Eynde, F. Brasseur, and T. Boon. 2002. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol. Rev. 188:51–64. - PubMed
-
- Coulie, P.G., and P. van der Bruggen. 2003. T-cell responses of vaccinated cancer patients. Curr. Opin. Immunol. 15:131–137. - PubMed
-
- Parmiani, G., C. Castelli, P. Dalerba, R. Mortarini, L. Rivoltini, F.M. Marincola, and A. Anichini. 2002. Cancer immunotherapy with peptide-based vaccines: what have we achieved? Where are we going? J. Natl. Cancer Inst. 94:805–818. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

