A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes
- PMID: 15824136
- PMCID: PMC2171904
- DOI: 10.1083/jcb.200409140
A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes
Abstract
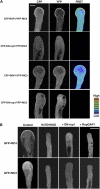
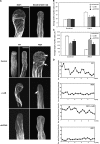
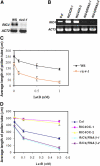
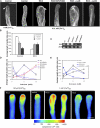
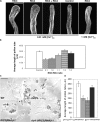
Tip growth in neuronal cells, plant cells, and fungal hyphae is known to require tip-localized Rho GTPase, calcium, and filamentous actin (F-actin), but how they interact with each other is unclear. The pollen tube is an exciting model to study spatiotemporal regulation of tip growth and F-actin dynamics. An Arabidopsis thaliana Rho family GTPase, ROP1, controls pollen tube growth by regulating apical F-actin dynamics. This paper shows that ROP1 activates two counteracting pathways involving the direct targets of tip-localized ROP1: RIC3 and RIC4. RIC4 promotes F-actin assembly, whereas RIC3 activates Ca(2+) signaling that leads to F-actin disassembly. Overproduction or depletion of either RIC4 or RIC3 causes tip growth defects that are rescued by overproduction or depletion of RIC3 or RIC4, respectively. Thus, ROP1 controls actin dynamics and tip growth through a check and balance between the two pathways. The dual and antagonistic roles of this GTPase may provide a unifying mechanism by which Rho modulates various processes dependent on actin dynamics in eukaryotic cells.
Figures
References
-
- Aspenstrom, P. 1999. Effectors of the Rho GTPases. Curr. Opin. Cell Biol. 11:95–102. - PubMed
-
- Bibikova, T.N., A. Zhigilei, and S. Gilroy. 1997. Root hair growth in Arabidopsis thaliana is directed by calcium and an endogenous polarity. Planta. 203:495–505. - PubMed
-
- Burbelo, P.D., D. Drechsel, and A. Hall. 1995. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J. Biol. Chem. 270:29071–29074. - PubMed
-
- Dickson, B.J. 2001. Rho GTPases in growth cone guidance. Curr. Opin. Neurobiol. 11:103–110. - PubMed
-
- Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature. 420:629–635. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

