Herpes simplex virus 1 infected cell protein 0 forms a complex with CIN85 and Cbl and mediates the degradation of EGF receptor from cell surfaces
- PMID: 15824310
- PMCID: PMC556299
- DOI: 10.1073/pnas.0501253102
Herpes simplex virus 1 infected cell protein 0 forms a complex with CIN85 and Cbl and mediates the degradation of EGF receptor from cell surfaces
Abstract
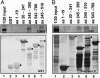
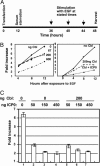
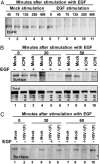
Infected cell protein 0 (ICP0) is a 775-residue multifunctional herpes simplex virus protein associated with numerous functions related to transactivation of gene expression and repression of host defenses to infection. We report that an uncharted domain of ICP0 located between residues 245 and 510 contains multiple SH3 domain binding motifs similar to those required for binding to CIN85, the M(r) 85,000 protein that interacts with Cbl. CIN85 and Cbl are involved in endocytosis and negative regulation of numerous receptor tyrosine kinases. We report that ICP0 binds CIN85 in a reciprocal manner and that the complexes pulled down by ICP0 also contain Cbl. We tested the role of ICP0 in the down-regulation of receptor tyrosine kinases by using epidermal growth factor receptor (EGFR) as a prototypic receptor. In transfection assays, ICP0, in the absence of other viral genes, down-regulated EGF-dependent expression of a reporter gene (luciferase). ICP0 also down-regulated both total and cell surface levels of EGFR in EGF-independent manner. In wild-type virus-infected cells, the surface levels of EGFR were also decreased in the absence of EGF stimulation. Stimulation by EGF enhanced the decrease in surface EGFR. We conclude that ICP0 encodes SH3 domain binding sites that function to down-regulate signaling pathways associated with receptor tyrosine kinases. The results suggest that ICP0 precludes signaling to the infected cells through the receptor tyrosine kinases.
Figures


Similar articles
-
Cbl E3 Ligase Mediates the Removal of Nectin-1 from the Surface of Herpes Simplex Virus 1-Infected Cells.J Virol. 2017 May 26;91(12):e00393-17. doi: 10.1128/JVI.00393-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28381567 Free PMC article.
-
Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors.Nature. 2002 Mar 14;416(6877):183-7. doi: 10.1038/416183a. Nature. 2002. PMID: 11894095
-
Alix/AIP1 antagonizes epidermal growth factor receptor downregulation by the Cbl-SETA/CIN85 complex.Mol Cell Biol. 2004 Oct;24(20):8981-93. doi: 10.1128/MCB.24.20.8981-8993.2004. Mol Cell Biol. 2004. PMID: 15456872 Free PMC article.
-
[Ubiquitination-mediated degradation of epidermal growth factor receptor].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2005 Feb;27(1):120-7. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2005. PMID: 15782507 Review. Chinese.
-
[Cbl--a polyfunctional regulator of cellular processes].Tsitologiia. 2003;45(11):1134-48. Tsitologiia. 2003. PMID: 14989153 Review. Russian.
Cited by
-
Widely Used Herpes Simplex Virus 1 ICP0 Deletion Mutant Strain dl1403 and Its Derivative Viruses Do Not Express Glycoprotein C Due to a Secondary Mutation in the gC Gene.PLoS One. 2015 Jul 17;10(7):e0131129. doi: 10.1371/journal.pone.0131129. eCollection 2015. PLoS One. 2015. PMID: 26186447 Free PMC article.
-
Role of Virus-Induced EGFR Trafficking in Proviral Functions.Biomolecules. 2023 Dec 9;13(12):1766. doi: 10.3390/biom13121766. Biomolecules. 2023. PMID: 38136637 Free PMC article. Review.
-
Opposing Regulation of the EGF Receptor: A Molecular Switch Controlling Cytomegalovirus Latency and Replication.PLoS Pathog. 2016 May 24;12(5):e1005655. doi: 10.1371/journal.ppat.1005655. eCollection 2016 May. PLoS Pathog. 2016. PMID: 27218650 Free PMC article.
-
Comprehensive proteomic analysis of influenza virus polymerase complex reveals a novel association with mitochondrial proteins and RNA polymerase accessory factors.J Virol. 2011 Sep;85(17):8569-81. doi: 10.1128/JVI.00496-11. Epub 2011 Jun 29. J Virol. 2011. PMID: 21715506 Free PMC article.
-
Cbl E3 Ligase Mediates the Removal of Nectin-1 from the Surface of Herpes Simplex Virus 1-Infected Cells.J Virol. 2017 May 26;91(12):e00393-17. doi: 10.1128/JVI.00393-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28381567 Free PMC article.
References
-
- Kurakin, A. V., Wu, S. & Bredesen, D. E. (2003) J. Biol. Chem. 278, 34102–34109. - PubMed
-
- Thien, C. B. & Langdon, W. Y. (2001) Nat. Rev. Mol. Cell Biol. 2, 294–307. - PubMed
-
- Dikic, I. & Giordano, S. (2003) Curr. Opin. Cell Biol. 15, 128–135. - PubMed
-
- Soubeyran, P., Kowanetz, K., Szymkiewicz, I., Langdon, W. Y. & Dikic, I. (2002) Nature 416, 183–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA 83939/CA/NCI NIH HHS/United States
- R01 CA078766/CA/NCI NIH HHS/United States
- NS 33376/NS/NINDS NIH HHS/United States
- CA 87661/CA/NCI NIH HHS/United States
- R01 NS045093/NS/NINDS NIH HHS/United States
- P01 CA087661/CA/NCI NIH HHS/United States
- R01 NS033376/NS/NINDS NIH HHS/United States
- P01 CA071933/CA/NCI NIH HHS/United States
- CA 71933/CA/NCI NIH HHS/United States
- R01 CA088860/CA/NCI NIH HHS/United States
- R37 CA078766/CA/NCI NIH HHS/United States
- CA 78766/CA/NCI NIH HHS/United States
- R01 CA083939/CA/NCI NIH HHS/United States
- CA 88860/CA/NCI NIH HHS/United States
- NS 45093/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

