Vocal experimentation in the juvenile songbird requires a basal ganglia circuit
- PMID: 15826219
- PMCID: PMC1069649
- DOI: 10.1371/journal.pbio.0030153
Vocal experimentation in the juvenile songbird requires a basal ganglia circuit
Abstract
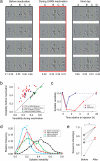
Songbirds learn their songs by trial-and-error experimentation, producing highly variable vocal output as juveniles. By comparing their own sounds to the song of a tutor, young songbirds gradually converge to a stable song that can be a remarkably good copy of the tutor song. Here we show that vocal variability in the learning songbird is induced by a basal-ganglia-related circuit, the output of which projects to the motor pathway via the lateral magnocellular nucleus of the nidopallium (LMAN). We found that pharmacological inactivation of LMAN dramatically reduced acoustic and sequence variability in the songs of juvenile zebra finches, doing so in a rapid and reversible manner. In addition, recordings from LMAN neurons projecting to the motor pathway revealed highly variable spiking activity across song renditions, showing that LMAN may act as a source of variability. Lastly, pharmacological blockade of synaptic inputs from LMAN to its target premotor area also reduced song variability. Our results establish that, in the juvenile songbird, the exploratory motor behavior required to learn a complex motor sequence is dependent on a dedicated neural circuit homologous to cortico-basal ganglia circuits in mammals.
Figures



References
-
- Sutton RS, Barto AG. Reinforcement learning: An introduction. Cambridge: MIT Press; 1998. 322 pp.
-
- Immelmann K. Song development in the zebra finch and other estrilid finches. In: Hinde RA, editor. Bird vocalizations. New York: Cambridge Univ. Press; 1969. pp. 61–74.
-
- Konishi M. The role of auditory feedback in the control of vocalizations in the white-crowned sparrow. Z Tierpsychol. 1965;22:770–783. - PubMed
-
- Nottebohm F, Kelley DB, Paton JA. Connections of vocal control nuclei in the canary telencephalon. J Comp Neurol. 1982;207:344–357. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

