Myeloid antigens in childhood lymphoblastic leukemia: clinical data point to regulation of CD66c distinct from other myeloid antigens
- PMID: 15826304
- PMCID: PMC1112585
- DOI: 10.1186/1471-2407-5-38
Myeloid antigens in childhood lymphoblastic leukemia: clinical data point to regulation of CD66c distinct from other myeloid antigens
Abstract
Background: Aberrant expression of myeloid antigens (MyAgs) on acute lymphoblastic leukemia (ALL) cells is a well-documented phenomenon, although its regulating mechanisms are unclear. MyAgs in ALL are interpreted e.g. as hallmarks of early differentiation stage and/or lineage indecisiveness. Granulocytic marker CD66c -- Carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) is aberrantly expressed on ALL with strong correlation to genotype (negative in TEL/AML1 and MLL/AF4, positive in BCR/ABL and hyperdiploid cases).
Methods: In a cohort of 365 consecutively diagnosed Czech B-precursor ALL patients, we analyze distribution of MyAg+ cases and mutual relationship among CD13, CD15, CD33, CD65 and CD66c. The most frequent MyAg (CD66c) is studied further regarding its stability from diagnosis to relapse, prognostic significance and regulation of surface expression. For the latter, flow cytometry, Western blot and quantitative RT-PCR on sorted cells is used.
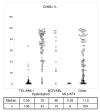
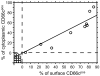
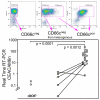
Results: We show CD66c is expressed in 43% patients, which is more frequent than other MyAgs studied. In addition, CD66c expression negatively correlates with CD13 (p < 0.0001), CD33 (p = 0.002) and/or CD65 (p = 0.029). Our data show that different myeloid antigens often differ in biological importance, which may be obscured by combining them into "MyAg positive ALL". We show that unlike other MyAgs, CD66c expression is not shifted from the onset of ALL to relapse (n = 39, time to relapse 0.3-5.3 years). Although opposite has previously been suggested, we show that CEACAM6 transcription is invariably followed by surface expression (by quantitative RT-PCR on sorted cells) and that malignant cells containing CD66c in cytoplasm without surface expression are not found by flow cytometry nor by Western blot in vivo. We report no prognostic significance of CD66c, globally or separately in genotype subsets of B-precursor ALL, nor an association with known risk factors (n = 254).
Conclusion: In contrast to general notion we show that different MyAgs in lymphoblastic leukemia represent different biological circumstances. We chose the most frequent and tightly genotype-associated MyAg CD66c to show its stabile expression in patients from diagnosis to relapse, which differs from what is known on the other MyAgs. Surface expression of CD66c is regulated at the gene transcription level, in contrast to previous reports.
Figures


Similar articles
-
CD66c expression in B-cell acute lymphoblastic leukemia: strength and weakness.Int J Lab Hematol. 2011 Feb;33(1):92-6. doi: 10.1111/j.1751-553X.2010.01254.x. Int J Lab Hematol. 2011. PMID: 20666852
-
[Expression of CD66c (CEACM6) in adult acute leukemia and its significance].Zhonghua Xue Ye Xue Za Zhi. 2006 Jun;27(6):370-3. Zhonghua Xue Ye Xue Za Zhi. 2006. PMID: 17147224 Chinese.
-
Significance of CD66c expression in childhood acute lymphoblastic leukemia.Leuk Res. 2014 Jan;38(1):42-8. doi: 10.1016/j.leukres.2013.10.008. Epub 2013 Oct 22. Leuk Res. 2014. PMID: 24231528
-
[Immunophenotyping of acute lymphatic leukemia: diagnostic aspects and clinical relevance].Wien Klin Wochenschr. 1994;106(8):231-2, 233-7. Wien Klin Wochenschr. 1994. PMID: 8023515 Review. German.
-
Review of the incidence and clinical relevance of myeloid antigen-positive acute lymphoblastic leukemia.Leukemia. 1991 Aug;5(8):637-45. Leukemia. 1991. PMID: 1886419 Review.
Cited by
-
EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes.Leukemia. 2012 Sep;26(9):1908-75. doi: 10.1038/leu.2012.120. Epub 2012 May 3. Leukemia. 2012. PMID: 22552007 Free PMC article.
-
The immunophenotype of T-lymphoblastic lymphoma in children and adolescents: a Children's Oncology Group report.Br J Haematol. 2012 Nov;159(4):454-61. doi: 10.1111/bjh.12042. Epub 2012 Sep 21. Br J Haematol. 2012. PMID: 22994934 Free PMC article.
-
Updating recommendations of the Brazilian Group of Flow Cytometry (GBCFLUX) for diagnosis of acute leukemias using four-color flow cytometry panels.Hematol Transfus Cell Ther. 2021 Oct-Dec;43(4):499-506. doi: 10.1016/j.htct.2021.04.001. Epub 2021 May 21. Hematol Transfus Cell Ther. 2021. PMID: 34127423 Free PMC article.
-
Prognostic value of the immune target CEACAM6 in cancer: a meta-analysis.Ther Adv Med Oncol. 2022 Jan 19;14:17588359211072621. doi: 10.1177/17588359211072621. eCollection 2022. Ther Adv Med Oncol. 2022. PMID: 35082925 Free PMC article.
-
Mechanisms of Immune Evasion in Acute Lymphoblastic Leukemia.Cancers (Basel). 2021 Mar 26;13(7):1536. doi: 10.3390/cancers13071536. Cancers (Basel). 2021. PMID: 33810515 Free PMC article. Review.
References
-
- Markert CL. Neoplasia: a disease of cell differentiation. Cancer Res. 1968;28:1908–1914. - PubMed
-
- Greaves MF. Differentiation-linked leukemogenesis in lymphocytes. Science. 1986;234:697–704. - PubMed
-
- Mori T, Sugita K, Suzuki T, Okazaki T, Manabe A, Hosoya R, Mizutani S, Kinoshita A, Nakazawa S. A novel monoclonal antibody, KOR-SA3544 which reacts to Philadelphia chromosome-positive acute lymphoblastic leukemia cells with high sensitivity. Leukemia. 1995;9:1233–1239. - PubMed
-
- Hrusak O, Trka J, Zuna J, Houskova J, Bartunkova J, Stary J. Aberrant expression of KOR-SA3544 antigen in childhood acute lymphoblastic leukemia predicts TEL-AML1 negativity. The Pediatric Hematology Working Group in the Czech Republic. Leukemia. 1998;12:1064–1070. doi: 10.1038/sj.leu.2401072. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

