Proliferation dynamics of germinative zone cells in the intact and excitotoxically lesioned postnatal rat brain
- PMID: 15826306
- PMCID: PMC1087489
- DOI: 10.1186/1471-2202-6-26
Proliferation dynamics of germinative zone cells in the intact and excitotoxically lesioned postnatal rat brain
Abstract
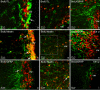
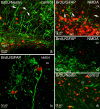
Background: The forebrain subventricular zone (SVZ)-olfactory bulb pathway and hippocampal subgranular zone (SGZ) generate neurons into adulthood in the mammalian brain. Neurogenesis increases after injury to the adult brain, but few studies examine the effect of injury on neural and glial precursors in the postnatal brain. To characterize the spatio-temporal dynamics of cell proliferation in the germinative zones, this study utilized a model of postnatal damage induced by NMDA injection in the right sensorimotor cortex at postnatal day 9. Dividing cell populations were labeled with 5-Bromodeoxyuridine (BrdU) in the intact and damaged postnatal brain. Identity of proliferating cells was determined by double immunolabeling with nestin, GFAP, NeuN and tomato lectin (TL).
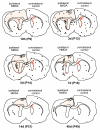
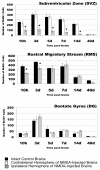
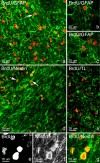
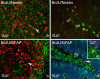
Results: In the control brain, grouped BrdU+ cells were observed in the Rostral Migratory Stream (RMS), SVZ and SGZ. Maximal proliferation was seen at P12, persisted until P23 and diminished by P49. After injury, a striking reduction in the number of BrdU+ cells was observed in the ipsilateral SVZ from 10 hours (58% decrease) until 14 days post-lesion (88% decrease). In contrast, an increase in grouped BrdU+ cells was seen in the striatum adjacent to the depleted SVZ. Significantly reduced numbers of BrdU+ cells were also seen in the RMS until 3 days post-lesion. No changes were noted in the SGZ. Both in controls and lesioned hemispheres, BrdU+ cells located in the germinal zones were mostly nestin positive and negative for GFAP, NeuN, and TL. In the SVZ area lining the ventricle, BrdU+/nestin+ cells were mainly located between TL+ ependyma and parenchymal GFAP+ astrocytes. After excitotoxicity, a decrease in the number and orientation of GFAP/nestin+ prolongations leaving the SVZ to the cortex, corpus callosum and striatum was noted until 5 days post-lesion.
Conclusion: Postnatal excitotoxic injury differentially affects proliferating cells in the germinative zones: no change is observed in the dentate gyrus whereas excitotoxicity causes a significant decrease in proliferating cells in the SVZ and RMS. Depletion of BrdU+ cells in the postnatal SVZ and RMS differs from previous studies after adult brain injury and may affect the SVZ-RMS migration and is suggestive of progenitor recruitment to injured areas.
Figures


Similar articles
-
Substantial migration of SVZ cells to the cortex results in the generation of new neurons in the excitotoxically damaged immature rat brain.Mol Cell Neurosci. 2008 Jun;38(2):170-82. doi: 10.1016/j.mcn.2008.02.002. Epub 2008 Mar 4. Mol Cell Neurosci. 2008. PMID: 18434192
-
Antioxidant Cu/Zn SOD: expression in postnatal brain progenitor cells.Neurosci Lett. 2006 Jun 19;401(1-2):71-6. doi: 10.1016/j.neulet.2006.03.010. Epub 2006 Mar 29. Neurosci Lett. 2006. PMID: 16567040
-
Neurogenesis in the striatum of the quinolinic acid lesion model of Huntington's disease.Neuroscience. 2004;127(2):319-32. doi: 10.1016/j.neuroscience.2004.04.061. Neuroscience. 2004. PMID: 15262322
-
Locating and labeling neural stem cells in the brain.J Cell Physiol. 2011 Jan;226(1):1-7. doi: 10.1002/jcp.22319. J Cell Physiol. 2011. PMID: 20658538 Review.
-
Neurogenesis and progenitor cells in the adult human brain: a comparison between hippocampal and subventricular progenitor proliferation.Dev Neurobiol. 2012 Jul;72(7):990-1005. doi: 10.1002/dneu.22028. Dev Neurobiol. 2012. PMID: 22539366 Review.
Cited by
-
Restoration of nigrostriatal dopamine neurons in post-MPTP treatment by the novel multifunctional brain-permeable iron chelator-monoamine oxidase inhibitor drug, M30.Neurotox Res. 2010 Jan;17(1):15-27. doi: 10.1007/s12640-009-9070-9. Epub 2009 Jul 16. Neurotox Res. 2010. PMID: 19609632
-
Disruption of wave-associated Rac GTPase-activating protein (Wrp) leads to abnormal adult neural progenitor migration associated with hydrocephalus.J Biol Chem. 2012 Nov 9;287(46):39263-74. doi: 10.1074/jbc.M112.398834. Epub 2012 Sep 24. J Biol Chem. 2012. PMID: 23007397 Free PMC article.
-
Modulation of neural stem/progenitor cell proliferation during experimental Herpes Simplex encephalitis is mediated by differential FGF-2 expression in the adult brain.Neurobiol Dis. 2013 Oct;58:144-55. doi: 10.1016/j.nbd.2013.05.018. Epub 2013 Jun 5. Neurobiol Dis. 2013. PMID: 23748078 Free PMC article.
-
Effect of neonatal injections of the neuropeptide Semax on cell proliferation in hippocampal dentate area in rats of two genotypes.Dokl Biol Sci. 2009 Jan-Feb;424:78-80. doi: 10.1134/s0012496609010232. Dokl Biol Sci. 2009. PMID: 19341092 No abstract available.
-
The number of proliferating cells in the rostral migratory stream of rat during the first postnatal month.Cell Mol Neurobiol. 2006 Oct-Nov;26(7-8):1453-61. doi: 10.1007/s10571-006-9039-7. Epub 2006 Apr 22. Cell Mol Neurobiol. 2006. PMID: 16633894 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

