Preferential suppression of trisomy 8 compared with normal hematopoietic cell growth by autologous lymphocytes in patients with trisomy 8 myelodysplastic syndrome
- PMID: 15827127
- PMCID: PMC1895154
- DOI: 10.1182/blood-2004-05-2017
Preferential suppression of trisomy 8 compared with normal hematopoietic cell growth by autologous lymphocytes in patients with trisomy 8 myelodysplastic syndrome
Abstract
Clinical observations and experimental evidence link bone marrow failure in myelodysplastic syndrome (MDS) with a T cell-dominated autoimmune process. Immunosuppressive therapy is effective in improving cytopenias in selected patients. Trisomy 8 is a frequent cytogenetic abnormality in bone marrow cells in patients with MDS, and its presence has been associated anecdotally with good response to immunotherapy. We studied 34 patients with trisomy 8 in bone marrow cells, some of whom were undergoing treatment with antithymocyte globulin (ATG). All had significant CD8+ T-cell expansions of one or more T-cell receptor (TCR) Vbeta subfamilies, as measured by flow cytometry; expanded subfamilies showed CDR3 skewing by spectratyping. Sorted T cells of the expanded Vbeta subfamilies, but not of the remaining subfamilies, inhibited trisomy 8 cell growth in short-term hematopoietic culture. The negative effects of Vbeta-expanded T cells were inhibited by major histocompatibility complex (MHC) class 1 monoclonal antibody (mAb) and Fas antagonist and required direct cell-to-cell contact. Sixty-seven percent of patients who had de novo MDS with trisomy 8 as the sole karyotypic abnormality responded to ATG with durable reversal of cytopenias and restoration of transfusion independence, with stable increase in the proportion of trisomy 8 bone marrow cells and normalization of the T-cell repertoire. An increased number of T cells with apparent specificity for trisomy 8 cells is consistent with an autoimmune pathophysiology in trisomy 8 MDS.
Figures
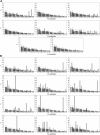



Comment in
-
Autoimmunity and Inflammation in Myelodysplastic Syndromes.Acta Haematol. 2016;136(2):108-17. doi: 10.1159/000446062. Epub 2016 Jun 24. Acta Haematol. 2016. PMID: 27337745
References
-
- Bennett JM. World Health Organization classification of the acute leukemias and myelodysplastic syndrome. Int J Hematol. 2000;72: 131-133. - PubMed
-
- Deeg HJ, Beckham C, Loken MR, et al. Negative regulators of hemopoiesis and stroma function in patients with myelodysplastic syndrome. Leuk Lymphoma. 2000;37: 405-414. - PubMed
-
- Parker JE, Fishlock KL, Mijovic A, et al.'Low-risk' myelodysplastic syndrome is associated with excessive apoptosis and an increased ratio of proversus anti-apoptotic bcl-2-related proteins. Br J Haematol. 1998;103: 1075-1082. - PubMed
-
- Parker JE, Mufti GJ. Ineffective haemopoiesis and apoptosis in myelodysplastic syndromes. Br J Haematol. 1998;101: 220-230. - PubMed
-
- Parker JE, Mufti GJ. Excessive apoptosis in low risk myelodysplastic syndromes (MDS). Leuk Lymphoma. 2000;40: 1-24. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

