Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells
- PMID: 15827136
- PMCID: PMC1895199
- DOI: 10.1182/blood-2004-11-4282
Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells
Abstract
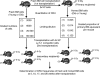
To test the hypothesis that aging has negative effects on stem-cell homing and engraftment, young or old C57BL/6 bone marrow (BM) cells were injected, using a limiting-dilution, competitive transplantation method, into old or young Ly5 congenic mice. Numbers of hematopoietic stem cells (HSCs) and progenitor cells (HPCs) recovered from BM or spleen were measured and compared with the numbers initially transplanted. Although the frequency of marrow competitive repopulation units (CRUs) increased approximately 2-fold from 2 months to 2 years of age, the BM homing efficiency of old CRUs was approximately 3-fold lower than that of young CRUs. Surprisingly, the overall size of individual stem-cell clones generated in recipients receiving a single CRU was not affected by donor age. However, the increased ages of HSC donors and HSC transplant recipients caused marked skewing of the pattern of engraftment toward the myeloid lineage, indicating that HSC-intrinsic and HSC-extrinsic (microenvironmental) age-related changes favor myelopoiesis. This correlated with changes after transplantation in the rate of recovery of circulating leukocytes, erythrocytes, and platelets. Recovery of the latter was especially blunted in aged recipients. Collectively, these findings may have implications for clinical HSC transplantation in which older persons increasingly serve as donors for elderly patients.
Figures
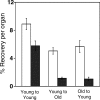
 ), and old cells into young recipients (▪)—are depicted. Data for young-to-old and old-to-young groups are derived from the experiments described in Tables 1 and 2. Data for the young-to-young group, though collected contemporaneously with those for the other transplantation groups, were reported previously by Szilvassy et al and are shown for comparison. All values are mean ± SEM, and statistically significant differences are designated with an asterisk (P < .05).
), and old cells into young recipients (▪)—are depicted. Data for young-to-old and old-to-young groups are derived from the experiments described in Tables 1 and 2. Data for the young-to-young group, though collected contemporaneously with those for the other transplantation groups, were reported previously by Szilvassy et al and are shown for comparison. All values are mean ± SEM, and statistically significant differences are designated with an asterisk (P < .05).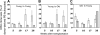
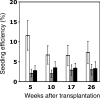
 ) CRUs as a function of donor and recipient age are represented as the mean ± SEM percentage of donor (Ly-5.2+)-derived PB leukocytes assessed 5 to 26 weeks after transplantation (4-8 mice per group from 2 pooled experiments). Note that data for the young-to-young group were collected contemporaneously with those for the other groups but were, in part, reported in Szilvassy et al.
) CRUs as a function of donor and recipient age are represented as the mean ± SEM percentage of donor (Ly-5.2+)-derived PB leukocytes assessed 5 to 26 weeks after transplantation (4-8 mice per group from 2 pooled experiments). Note that data for the young-to-young group were collected contemporaneously with those for the other groups but were, in part, reported in Szilvassy et al.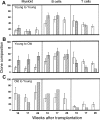
 ) CRU were identified as described in the Figure 4 legend and in “Materials and methods.” The lineage composition of clones generated by single, young, or old HSCs in old or young hosts was defined as the proportion of donor (Ly-5.2+)-derived leukocytes expressing markers for B (CD45R/B220+) or T (CD90/Thy-1.2+) lymphocytes or for myeloid (Gr-1/Ly6G+ and Mac-1/CD11b+) cells. Values shown for the 3 combinations of variably aged donors and recipients represent the mean ± SEM of 2 pooled experiments with 3 to 6 mice per group. Note that data for the young-to-young group were collected contemporaneously with those for other groups but were, in part, reported in Szilvassy et al.
) CRU were identified as described in the Figure 4 legend and in “Materials and methods.” The lineage composition of clones generated by single, young, or old HSCs in old or young hosts was defined as the proportion of donor (Ly-5.2+)-derived leukocytes expressing markers for B (CD45R/B220+) or T (CD90/Thy-1.2+) lymphocytes or for myeloid (Gr-1/Ly6G+ and Mac-1/CD11b+) cells. Values shown for the 3 combinations of variably aged donors and recipients represent the mean ± SEM of 2 pooled experiments with 3 to 6 mice per group. Note that data for the young-to-young group were collected contemporaneously with those for other groups but were, in part, reported in Szilvassy et al.
 ) CRU were identified as described in the Figure 4 legend and in “Materials and methods.” The differential contribution of individual CRUs to engraftment was defined as the proportion of all circulating B (CD45R/B220+) or T (CD90/Thy-1.2+) lymphocytes or myeloid (Gr-1/Ly6G+ and Mac-1/CD11b+) cells that were Ly-5.2+. Values shown for the 3 combinations of variably aged donors and recipients represent the mean ± SEM of 2 pooled experiments with 3 to 6 mice per group. Note that data for the young-to-young group were collected contemporaneously with those for other groups but were, in part, reported in Szilvassy et al.
) CRU were identified as described in the Figure 4 legend and in “Materials and methods.” The differential contribution of individual CRUs to engraftment was defined as the proportion of all circulating B (CD45R/B220+) or T (CD90/Thy-1.2+) lymphocytes or myeloid (Gr-1/Ly6G+ and Mac-1/CD11b+) cells that were Ly-5.2+. Values shown for the 3 combinations of variably aged donors and recipients represent the mean ± SEM of 2 pooled experiments with 3 to 6 mice per group. Note that data for the young-to-young group were collected contemporaneously with those for other groups but were, in part, reported in Szilvassy et al.
References
-
- Szilvassy SJ. The biology of hematopoietic stem cells. Arch Med Res. 2003;34: 446-460. - PubMed
-
- Geiger H, Van Zant G. The aging of lympho-hematopoietic stem cells. Nat Immunol. 2002;3: 329-333. - PubMed
-
- Van Zant G, Liang Y. The role of stem cells in aging. Exp Hematol. 2003;31: 659-672. - PubMed
-
- de Haan G, Van Zant G. Dynamic changes in mouse hematopoietic stem cell numbers during aging. Blood. 1999;93: 3294-3301. - PubMed
-
- Geiger H, True JM, de Haan G, Van Zant G. Age- and stage-specific regulation patterns in the hematopoietic stem cell hierarchy. Blood. 2001;98: 2966-2972. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

