RhoA signaling is required for respiratory syncytial virus-induced syncytium formation and filamentous virion morphology
- PMID: 15827147
- PMCID: PMC1082718
- DOI: 10.1128/JVI.79.9.5326-5336.2005
RhoA signaling is required for respiratory syncytial virus-induced syncytium formation and filamentous virion morphology
Abstract
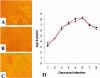
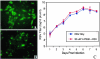
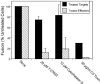
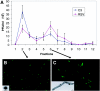
Respiratory syncytial virus (RSV) is an important human pathogen that can cause severe and life-threatening respiratory infections in infants, the elderly, and immunocompromised adults. RSV infection of HEp-2 cells induces the activation of RhoA, a small GTPase. We therefore asked whether RhoA signaling is important for RSV replication or syncytium formation. The treatment of HEp-2 cells with Clostridium botulinum C3, an enzyme that ADP-ribosylates and specifically inactivates RhoA, inhibited RSV-induced syncytium formation and cell-to-cell fusion, although similar levels of PFU were released into the medium and viral protein expression levels were equivalent. Treatment with another inhibitor of RhoA signaling, the Rho kinase inhibitor Y-27632, yielded similar results. Scanning electron microscopy of C3-treated infected cells showed reduced numbers of single blunted filaments, in contrast to the large clumps of long filaments in untreated infected cells. These data suggest that RhoA signaling is associated with filamentous virus morphology, cell-to-cell fusion, and syncytium formation but is dispensable for the efficient infection and production of infectious virus in vitro. Next, we developed a semiquantitative method to measure spherical and filamentous virus particles by using sucrose gradient velocity sedimentation. Fluorescence and transmission electron microscopy confirmed the separation of spherical and filamentous forms of infectious virus into two identifiable peaks. The C3 treatment of RSV-infected cells resulted in a shift to relatively more spherical virions than those from untreated cells. These data suggest that viral filamentous protuberances characteristic of RSV infection are associated with RhoA signaling, are important for filamentous virion morphology, and may play a role in initiating cell-to-cell fusion.
Figures



References
-
- Bergelson, J. M., and R. W. Finberg. 1993. Integrins as receptors for virus attachment and cell entry. Trends Microbiol. 1:287-288. - PubMed
-
- Brown, G., H. W. Rixon, and R. J. Sugrue. 2002. Respiratory syncytial virus assembly occurs in GM1-rich regions of the host-cell membrane and alters the cellular distribution of tyrosine-phosphorylated caveolin-1. J. Gen. Virol. 83:1841-1850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

