Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes
- PMID: 15827153
- PMCID: PMC1082762
- DOI: 10.1128/JVI.79.9.5386-5399.2005
Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes
Abstract
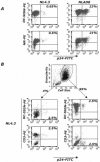
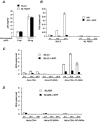
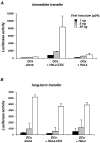
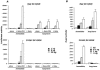
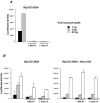
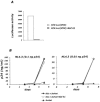
HIV-1 virions are efficiently captured by monocyte-derived immature dendritic cells (iDCs), as well as by cell lines expressing the lectin DC-SIGN. Viral infectivity can be retained for several days, and even enhanced, before transmission to CD4+ lymphocytes. The role of DC-SIGN in viral retention and enhancement of infection is not fully understood and varies according to the cell line expressing the lectin. We studied here the mechanisms underlying this process. We focused our study on X4-tropic human immunodeficiency virus (HIV) strains, since they were widely believed not to replicate in iDCs. However, we first show that X4 HIV replicates covertly and slowly in iDCs. This is also the case in Raji-DC-SIGN cells, which are classically used to study HIV transmission. We used either single-cycle or replicative HIV and measured viral RT and replication to further demonstrate that transfer of incoming virions from iDCs or DC-SIGN+ cells occurs only on the short-term (i.e., a few hours after viral exposure). There is no long-term storage of original HIV particles in these cells. A few days after viral exposure, replicative viruses, and not single-cycle virions, are transmitted to CD4+ cells. The cell-type-dependent activity of DC-SIGN reflects the ability of HIV to replicate covertly in some cells, and not in others.
Figures




References
-
- Arrighi, J. F., M. Pion, M. Wiznerowicz, T. B. Geijtenbeek, E. Garcia, S. Abraham, F. Leuba, V. Dutoit, O. Ducrey-Rundquist, Y. van Kooyk, D. Trono, and V. Piguet. 2004. Lentivirus-mediated RNA interference of DC-SIGN expression inhibits human immunodeficiency virus transmission from dendritic cells to T cells. J. Virol. 78:10848-10855. - PMC - PubMed
-
- Ayehunie, S., E. A. Garcia-Zepeda, J. A. Hoxie, R. Horuk, T. S. Kupper, A. D. Luster, and R. M. Ruprecht. 1997. Human immunodeficiency virus-1 entry into purified blood dendritic cells through CC and CXC chemokine coreceptors. Blood 90:1379-1386. - PubMed
-
- Bakri, Y., C. Schiffer, V. Zennou, P. Charneau, E. Kahn, A. Benjouad, J. C. Gluckman, and B. Canque. 2001. The maturation of dendritic cells results in postintegration inhibition of HIV-1 replication. J. Immunol. 166:3780-3788. - PubMed
-
- Baribaud, F., R. W. Doms, and S. Pohlmann. 2002. The role of DC-SIGN and DC-SIGNR in HIV and Ebola virus infection: can potential therapeutics block virus transmission and dissemination? Expert Opin. Ther. Targets 6:423-431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

