Recombinant, live-attenuated tetravalent dengue virus vaccine formulations induce a balanced, broad, and protective neutralizing antibody response against each of the four serotypes in rhesus monkeys
- PMID: 15827166
- PMCID: PMC1082773
- DOI: 10.1128/JVI.79.9.5516-5528.2005
Recombinant, live-attenuated tetravalent dengue virus vaccine formulations induce a balanced, broad, and protective neutralizing antibody response against each of the four serotypes in rhesus monkeys
Abstract
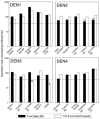
Three tetravalent vaccine (TV) formulations of previously described monovalent dengue (DEN) virus vaccine candidates were compared to a tetravalent formulation of wild-type DEN viruses (T-wt) for replication in SCID mice transplanted with human liver cells (SCID-HuH-7) or for replication and immunogenicity in rhesus monkeys. TV-1 consists of recombinant DEN1, -2, -3, and -4, each with a 30-nucleotide deletion in the 3' untranslated region (Delta30). TV-2 consists of rDEN1Delta30, rDEN4Delta30, and two antigenic chimeric viruses, rDEN2/4Delta30 and rDEN3/4Delta30, both also bearing the Delta30 mutation. TV-3 consists of rDEN1Delta30, rDEN2Delta30, rDEN4Delta30, and a 10-fold higher dose of rDEN3/4Delta30. TV-1 and TV-2 were attenuated in SCID-HuH-7 mice with minimal interference in replication among the virus components. TV-1, -2, and -3 were attenuated in rhesus monkeys as measured by duration and peak of viremia. Each monkey immunized with TV-1 and TV-3 seroconverted to the four DEN components by day 28 with neutralization titers ranging from 1:52 to 1:273 and 1:59 to 1:144 for TV-1 and TV-3, respectively. TV-2 induced low antibody titers to DEN2 and DEN3, but a booster immunization after 4 months increased the neutralizing antibody titers to greater than 1:100 against each serotype and elicited broad neutralizing activity against 19 of 20 DEN subtypes. A single dose of TV-2 induced protection against wild-type DEN1, DEN3, and DEN4 challenge, but not DEN2. However, two doses of TV-2 or TV-3 induced protection against DEN2 challenge. Two tetravalent formulations, TV-2 and TV-3, possess properties of a successful DEN vaccine and can be considered for evaluation in clinical trials.
Figures


References
-
- Almond, J., J. Clemens, H. Engers, S. Halstead, H. Khiem, A. Pablos-Mendez, Y. Pervikov, and T. Tram. 2002. Accelerating the development and introduction of a dengue vaccine for poor children, 5-8 December 2001, Ho Chi Minh City, VietNam. Vaccine 20:3043-3046. - PubMed
-
- Blaney, J. E., Jr., C. T. Hanson, C. Y. Firestone, K. A. Hanley, B. R. Murphy, and S. S. Whitehead. 2004. Genetically modified, live attenuated dengue virus type 3 vaccine candidates. Am. J. Trop. Med. Hyg. 71:811-821. - PubMed
-
- Blaney, J. E., Jr., D. H. Johnson, C. Y. Firestone, C. T. Hanson, B. R. Murphy, and S. S. Whitehead. 2001. Chemical mutagenesis of dengue virus type 4 yields mutant viruses which are temperature sensitive in Vero cells or human liver cells and attenuated in mice. J. Virol. 75:9731-9740. - PMC - PubMed
-
- Blaney, J. E., Jr., D. H. Johnson, G. G. Manipon, C. Y. Firestone, C. T. Hanson, B. R. Murphy, and S. S. Whitehead. 2002. Genetic basis of attenuation of dengue virus type 4 small plaque mutants with restricted replication in suckling mice and in SCID mice transplanted with human liver cells. Virology 300:125-139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

