ATPgammaS disrupts human immunodeficiency virus type 1 virion core integrity
- PMID: 15827170
- PMCID: PMC1082765
- DOI: 10.1128/JVI.79.9.5557-5567.2005
ATPgammaS disrupts human immunodeficiency virus type 1 virion core integrity
Abstract
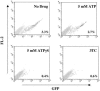
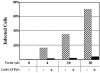
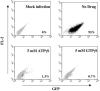
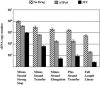
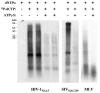
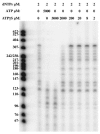
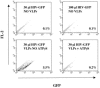
Heat shock protein 70 (Hsp70) is incorporated within the membrane of primate lentiviral virions. Here we demonstrate that Hsp70 is also incorporated into oncoretroviral virions and that it remains associated with membrane-stripped human immunodeficiency virus type 1 (HIV-1) virion cores. To determine if Hsp70 promotes virion infectivity, we attempted to generate Hsp70-deficient virions with gag deletion mutations, Hsp70 transdominant mutants, or RNA interference, but these efforts were confounded, largely because they disrupt virion assembly. Given that polypeptide substrates are bound and released by Hsp70 in an ATP-hydrolytic reaction cycle, we supposed that incubation of HIV-1 virions with ATP would perturb Hsp70 interaction with substrates in the virion and thereby decrease infectivity. Treatment with ATP or ADP had no observable effect, but ATPgammaS and GTPgammaS, nucleotide triphosphate analogues resistant to Hsp70 hydrolysis, dramatically reduced the infectivity of HIV-1 and murine leukemia virus virions. ATPgammaS-treated virions were competent for fusion with susceptible target cells, but viral cDNA synthesis was inhibited to an extent that correlated with the magnitude of decrease in infectivity. Intravirion reverse transcription by HIV-1, simian immunodeficiency virus, or murine leukemia virus was also inhibited by ATPgammaS. The effects of ATPgammaS on HIV-1 reverse transcription appeared to be indirect, resulting from disruption of virion core morphology that was evident by transmission electron microscopy. Consistent with effects on capsid conformation, ATPgammaS-treated viruslike particles failed to saturate host antiviral restriction activity. Our observations support a model in which the catalytic activity of virion-associated Hsp70 is required to maintain structural integrity of the virion core.
Figures



Similar articles
-
Cholesterol depletion of human immunodeficiency virus type 1 and simian immunodeficiency virus with beta-cyclodextrin inactivates and permeabilizes the virions: evidence for virion-associated lipid rafts.J Virol. 2003 Aug;77(15):8237-48. doi: 10.1128/jvi.77.15.8237-8248.2003. J Virol. 2003. PMID: 12857892 Free PMC article.
-
Perturbing HIV-1 Ribosomal Frameshifting Frequency Reveals a cis Preference for Gag-Pol Incorporation into Assembling Virions.J Virol. 2022 Jan 12;96(1):e0134921. doi: 10.1128/JVI.01349-21. Epub 2021 Oct 13. J Virol. 2022. PMID: 34643428 Free PMC article.
-
Morphologic changes in human immunodeficiency virus type 1 virions secondary to intravirion reverse transcription: evidence indicating that reverse transcription may not take place within the intact viral core.J Hum Virol. 2000 May-Jun;3(3):165-72. J Hum Virol. 2000. PMID: 10881997
-
Specific incorporation of heat shock protein 70 family members into primate lentiviral virions.J Virol. 2002 May;76(9):4666-70. doi: 10.1128/jvi.76.9.4666-4670.2002. J Virol. 2002. PMID: 11932435 Free PMC article.
-
Exploring HIV-1 Maturation: A New Frontier in Antiviral Development.Viruses. 2024 Sep 6;16(9):1423. doi: 10.3390/v16091423. Viruses. 2024. PMID: 39339899 Free PMC article. Review.
Cited by
-
Gene expression deregulation in postnatal skeletal muscle of TK2 deficient mice reveals a lower pool of proliferating myogenic progenitor cells.PLoS One. 2013;8(1):e53698. doi: 10.1371/journal.pone.0053698. Epub 2013 Jan 14. PLoS One. 2013. PMID: 23341978 Free PMC article.
-
Cell factors stimulate human immunodeficiency virus type 1 reverse transcription in vitro.J Virol. 2008 Feb;82(3):1425-37. doi: 10.1128/JVI.01808-07. Epub 2007 Nov 28. J Virol. 2008. PMID: 18045931 Free PMC article.
-
The retrovirus RNA trafficking granule: from birth to maturity.Retrovirology. 2006 Mar 17;3:18. doi: 10.1186/1742-4690-3-18. Retrovirology. 2006. PMID: 16545126 Free PMC article. Review.
-
Heat shock proteins and viral infection.Front Immunol. 2022 Aug 5;13:947789. doi: 10.3389/fimmu.2022.947789. eCollection 2022. Front Immunol. 2022. PMID: 35990630 Free PMC article. Review.
-
Cucumber Necrosis Virus Recruits Cellular Heat Shock Protein 70 Homologs at Several Stages of Infection.J Virol. 2015 Dec 30;90(7):3302-17. doi: 10.1128/JVI.02833-15. J Virol. 2015. PMID: 26719261 Free PMC article.
References
-
- Agostini, I., S. Popov, J. Li, L. Dubrovsky, T. Hao, and M. Bukrinsky. 2000. Heat-shock protein 70 can replace viral protein R of HIV-1 during nuclear import of the viral preintegration complex. Exp. Cell Res. 259:398-403. - PubMed
-
- Alce, T. M., and W. Popik. 2004. APOBEC3G is incorporated into virus-like particles by a direct interaction with HIV-1 Gag nucleocapsid protein. J. Biol. Chem. 279:34083-34086. - PubMed
-
- Asmal, M., J. Colgan, F. Naef, B. Yu, Y. Lee, M. Magnasco, and J. Luban. 2003. Production of ribosome components in effector CD4+ T cells is accelerated by TCR stimulation and coordinated by ERK-MAPK. Immunity 19:535-548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

