Macrophage inflammatory protein 1alpha inhibits postentry steps of human immunodeficiency virus type 1 infection via suppression of intracellular cyclic AMP
- PMID: 15827177
- PMCID: PMC1082740
- DOI: 10.1128/JVI.79.9.5625-5631.2005
Macrophage inflammatory protein 1alpha inhibits postentry steps of human immunodeficiency virus type 1 infection via suppression of intracellular cyclic AMP
Abstract
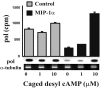
Primary isolates of human immunodeficiency virus type 1 (HIV-1) predominantly use chemokine receptor CCR5 to enter target cells. The natural ligands of CCR5, the beta-chemokines macrophage inflammatory protein 1alpha (MIP-1alpha), MIP-1beta, and RANTES, interfere with HIV-1 binding to CCR5 receptors and decrease the amount of virions entering cells. Although the inhibition of HIV-1 entry by beta-chemokines is well documented, their effects on postentry steps of the viral life cycle and on host cell components that control the outcome of infection after viral entry are not well defined. Here, we show that all three beta-chemokines, and MIP-1alpha in particular, inhibit postentry steps of the HIV-1 life cycle in primary lymphocytes, presumably via suppression of intracellular levels of cyclic AMP (cAMP). Productive HIV-1 infection of primary lymphocytes requires cellular activation. Cell activation increases intracellular cAMP, which is required for efficient synthesis of proviral DNA during early steps of viral infection. Binding of MIP-1alpha to cognate receptors decreases activation-induced intracellular cAMP levels through the activation of inhibitory G proteins. Furthermore, inhibition of one of the downstream targets of cAMP, cAMP-dependent PKA, significantly inhibits synthesis of HIV-1-specific DNA without affecting virus entry. These data reveal that beta-chemokine-mediated inhibition of virus replication in primary lymphocytes combines inhibitory effects at the entry and postentry levels and imply the involvement of beta-chemokine-induced signaling in postentry inhibition of HIV-1 infection.
Figures







References
-
- Aandahl, E. M., P. Aukrust, B. S. Skalhegg, F. Muller, S. S. Froland, V. Hansson, and K. Tasken. 1998. Protein kinase A type I antagonist restores immune responses of T cells from HIV-infected patients. FASEB J. 12:855-862. - PubMed
-
- Aukrust, P., E. M. Aandahl, B. S. Skalhegg, I. Nordoy, V. Hansson, K. Tasken, S. S. Froland, and F. Muller. 1999. Increased activation of protein kinase A type I contributes to the T cell deficiency in common variable immunodeficiency. J. Immunol. 162:1178-1185. - PubMed
-
- Blumer, K. J., and G. L. Johnson. 1994. Diversity in function and regulation of MAP kinase pathways. Trends Biochem. Sci. 19:236-240. - PubMed
-
- Buc, H. A., A. Moncion, M. Hamet, and J. L. Perignon. 1993. T-cell antigen receptor-mediated enhancement of the adenylate cyclase pathway depends on tyrosine protein kinases. Int. J. Immunopharmacol. 15:415-422. - PubMed
-
- Cartier, C., B. Hemonnot, B. Gay, M. Bardy, C. Sanchiz, C. Devaux, and L. Briant. 2003. Active cAMP-dependent protein kinase incorporated within highly purified HIV-1 particles is required for viral infectivity and interacts with viral capsid protein. J. Biol. Chem. 278:35211-35219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

