Peptide mimetics of gamma interferon possess antiviral properties against vaccinia virus and other viruses in the presence of poxvirus B8R protein
- PMID: 15827178
- PMCID: PMC1082775
- DOI: 10.1128/JVI.79.9.5632-5639.2005
Peptide mimetics of gamma interferon possess antiviral properties against vaccinia virus and other viruses in the presence of poxvirus B8R protein
Abstract
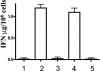
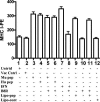
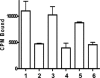
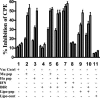
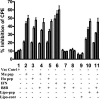
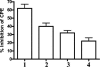
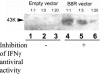
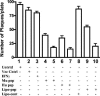
We have developed peptide mimetics of gamma interferon (IFN-gamma) that play a direct role in the activation and nuclear translocation of STAT1alpha transcription factor. These mimetics do not act through recognition by the extracellular domain of IFN-gamma receptor but rather bind to the cytoplasmic domain of the receptor chain 1, IFNGR-1, and thereby initiate the cellular signaling. Thus, we hypothesized that these mimetics would bypass the poxvirus virulence factor B8R protein that binds to intact IFN-gamma and prevents its interaction with the receptor. Human and murine IFN-gamma mimetic peptides were introduced into an adenoviral vector for intracellular expression. Murine IFN-gamma mimetic peptide was also expressed via chemical synthesis with an attached lipophilic group for penetration of cell plasma membrane. In contrast to intact human IFN-gamma, the mimetics did not bind poxvirus B8R protein, a homolog of the IFN-gamma receptor extracellular domain. Expression of B8R protein in WISH cells did not block the antiviral effect of the mimetics against encephalomyocarditis or vesicular stomatitis virus, while the antiviral activity of human IFN-gamma was neutralized. Consistent with the antiviral activity, the upregulation of MHC class I molecules on WISH cells by the IFN-gamma mimetics was not affected by B8R protein, while IFN-gamma-induced upregulation was blocked. Finally, the mimetics, but not IFN-gamma, inhibited vaccinia virus replication in African green monkey kidney BSC-40 cells. The data presented demonstrate that small peptide mimetics of IFN-gamma can avoid the B8R virulence factor for poxviruses and, thus, are potential candidates for antivirals against smallpox virus.
Figures
Similar articles
-
IFN mimetic as a therapeutic for lethal vaccinia virus infection: possible effects on innate and adaptive immune responses.J Immunol. 2007 Apr 1;178(7):4576-83. doi: 10.4049/jimmunol.178.7.4576. J Immunol. 2007. PMID: 17372016
-
Vaccinia virus-encoded cytokine receptor binds and neutralizes chicken interferon-gamma.Virology. 1998 Sep 1;248(2):231-40. doi: 10.1006/viro.1998.9278. Virology. 1998. PMID: 9721232
-
Vaccinia virus vectors with an inactivated gamma interferon receptor homolog gene (B8R) are attenuated In vivo without a concomitant reduction in immunogenicity.J Virol. 2001 Jan;75(1):11-8. doi: 10.1128/JVI.75.1.11-18.2001. J Virol. 2001. PMID: 11119568 Free PMC article.
-
Gamma interferon signaling: insights to development of interferon mimetics.Cell Mol Biol (Noisy-le-grand). 2006 May 15;52(1):71-6. Cell Mol Biol (Noisy-le-grand). 2006. PMID: 16914098 Review.
-
How Does Vaccinia Virus Interfere With Interferon?Adv Virus Res. 2018;100:355-378. doi: 10.1016/bs.aivir.2018.01.003. Epub 2018 Feb 16. Adv Virus Res. 2018. PMID: 29551142 Review.
Cited by
-
SOCS-1 mimetics protect mice against lethal poxvirus infection: identification of a novel endogenous antiviral system.J Virol. 2009 Feb;83(3):1402-15. doi: 10.1128/JVI.01138-08. Epub 2008 Nov 19. J Virol. 2009. PMID: 19019946 Free PMC article.
-
Interferon gamma is recognised by importin alpha/beta: enhanced nuclear localising and transactivation activities of an interferon gamma mimetic.FEBS Lett. 2008 May 14;582(11):1569-74. doi: 10.1016/j.febslet.2008.03.054. Epub 2008 Apr 9. FEBS Lett. 2008. PMID: 18405666 Free PMC article.
-
Corrigendum: Intrauterine Viral Infections: Impact of Inflammation on Fetal Neurodevelopment.Front Neurosci. 2021 Dec 10;15:817697. doi: 10.3389/fnins.2021.817697. eCollection 2021. Front Neurosci. 2021. PMID: 34955742 Free PMC article.
-
Type I interferon mimetics bypass vaccinia virus decoy receptor virulence factor for protection of mice against lethal infection.Clin Vaccine Immunol. 2014 Aug;21(8):1178-84. doi: 10.1128/CVI.00204-14. Epub 2014 Jun 25. Clin Vaccine Immunol. 2014. PMID: 24964806 Free PMC article.
-
Controlling Nuclear Jaks and Stats for Specific Gene Activation by Ifn γ and Other Cytokines: A Possible Steroid-like Connection.J Clin Cell Immunol. 2011 Sep 3;2(4):1000112. doi: 10.4172/2155-9899.1000112. J Clin Cell Immunol. 2011. PMID: 22924155 Free PMC article.
References
-
- Ahmed, C. M. I., K. N. Wills, B. J. Sugarman, D. E. Johnson, M. Ramachandra, T. L. Nagabhushan, and J. A. Howe. 2001. Selective expression of nonsecreted interferon by an adenoviral vector confers antiproliferative and antiviral properties and causes reduction of tumor growth in nude mice. J. Interferon Cytokine Res. 21:399-408. - PubMed
-
- Ahmed, C. M. I., M. A. Burkhart, M. G. Mujtaba, P. S. Subramaniam, and H. M. Johnson. 2003. The role of IFNγ nuclear localization sequence in intracellular function. J. Cell Sci. 116:3089-3098. - PubMed
-
- Alcami, A., and G. L. Smith. 2002. The vaccinia virus soluble interferon gamma receptor is a homodimer. J. Gen. Virol. 83:545-549. - PubMed
-
- Atlas, R. M. 2002. Bioterrorism: from threat to reality. Annu. Rev. Microbiol. 56:167-185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

