Binding and transfer of human immunodeficiency virus by DC-SIGN+ cells in human rectal mucosa
- PMID: 15827191
- PMCID: PMC1082722
- DOI: 10.1128/JVI.79.9.5762-5773.2005
Binding and transfer of human immunodeficiency virus by DC-SIGN+ cells in human rectal mucosa
Abstract
The role of DC-SIGN on human rectal mucosal dendritic cells is unknown. Using highly purified human rectal mucosal DC-SIGN+ cells and an ultrasensitive real-time reverse transcription-PCR assay to quantify virus binding, we found that HLA-DR+/DC-SIGN+ cells can bind and transfer more virus than the HLA-DR+/DC-SIGN- cells. Greater than 90% of the virus bound to total mucosal mononuclear cells (MMCs) was accounted for by the DC-SIGN+ cells, which comprise only 1 to 5% of total MMCs. Significantly, anti-DC-SIGN antibodies blocked 90% of the virus binding when more-physiologic amounts of virus inoculum were used. DC-SIGN expression in the rectal mucosa was significantly correlated with the interleukin-10 (IL-10)/IL-12 ratio (r = 0.58, P < 0.002; n = 26) among human immunodeficiency virus (HIV)-positive patients. Ex vivo and in vitro data implicate the role of IL-10 in upregulating DC-SIGN expression and downregulating expression of the costimulatory molecules CD80/CD86. Dendritic cells derived from monocytes (MDDCs) in the presence of IL-10 render the MDDCs less responsive to maturation stimuli, such as lipopolysaccharide and tumor necrosis factor alpha, and migration to the CCR7 ligand macrophage inflammatory protein 3beta. Thus, an increased IL-10 environment could render DC-SIGN(+) cells less immunostimulatory and migratory, thereby dampening an effective immune response. DC-SIGN and the IL-10/IL-12 axis may play significant roles in the mucosal transmission and pathogenesis of HIV type 1.
Figures
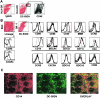

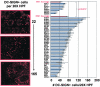

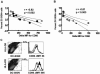
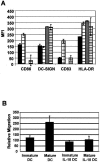
References
-
- Anton, P. A., M. A. Poles, J. Elliott, S. H. Mao, I. McGowan, H. J. Lenz, and I. S. Chen. 2001. Sensitive and reproducible quantitation of mucosal HIV-1 RNA and DNA viral burden in patients with detectable and undetectable plasma viral HIV-1 RNA using endoscopic biopsies. J. Virol. Methods 95:65-79. - PubMed
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. - PubMed
-
- Baribaud, F., S. Pohlmann, T. Sparwasser, M. T. Kimata, Y. K. Choi, B. S. Haggarty, N. Ahmad, T. Macfarlan, T. G. Edwards, G. J. Leslie, J. Arnason, T. A. Reinhart, J. T. Kimata, D. R. Littman, J. A. Hoxie, and R. W. Doms. 2001. Functional and antigenic characterization of human, rhesus macaque, pigtailed macaque, and murine DC-SIGN. J. Virol. 75:10281-10289. - PMC - PubMed
-
- Bell, S. J., R. Rigby, N. English, S. D. Mann, S. C. Knight, M. A. Kamm, and A. J. Stagg. 2001. Migration and maturation of human colonic dendritic cells. J. Immunol. 166:4958-4967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

