Torsin-mediated protection from cellular stress in the dopaminergic neurons of Caenorhabditis elegans
- PMID: 15829632
- PMCID: PMC6724936
- DOI: 10.1523/JNEUROSCI.5157-04.2005
Torsin-mediated protection from cellular stress in the dopaminergic neurons of Caenorhabditis elegans
Abstract
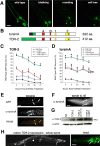
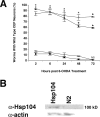
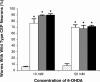
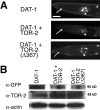
Parkinson's disease (PD) is linked genetically to proteins that function in the management of cellular stress resulting from protein misfolding and oxidative damage. Overexpression or mutation of alpha-synuclein results in the formation of Lewy bodies and neurodegeneration of dopaminergic (DA) neurons. Human torsinA, mutations in which cause another movement disorder termed early-onset torsion dystonia, is highly expressed in DA neurons and is also a component of Lewy bodies. Previous work has established torsins as having molecular chaperone activity. Thus, we examined the ability of torsinA to manage cellular stress within DA neurons of the nematode Caenorhabditis elegans. Worm DA neurons undergo a reproducible pattern of neurodegeneration after treatment with 6-hydroxydopamine (6-OHDA), a neurotoxin commonly used to model PD. Overexpression of torsins in C. elegans DA neurons results in dramatic suppression of neurodegeneration after 6-OHDA treatment. In contrast, expression of either dystonia-associated mutant torsinA or combined overexpression of wild-type and mutant torsinA yielded greatly diminished neuroprotection against 6-OHDA. We further demonstrated that torsins seem to protect DA neurons from 6-OHDA through downregulating protein levels of the dopamine transporter (DAT-1) in vivo. Additionally, we determined that torsins protect robustly against DA neurodegeneration caused by overexpression of alpha-synuclein. Using mutant nematodes lacking DAT-1 function, we also showed that torsin neuroprotection from alpha-synuclein-induced degeneration occurs in a manner independent of this transporter. Together, these data have mechanistic implications for movement disorders, because our results demonstrate that torsin proteins have the capacity to manage sources of cellular stress within DA neurons.
Figures


Similar articles
-
Acetaminophen attenuates dopamine neuron degeneration in animal models of Parkinson's disease.Neurosci Lett. 2008 Jul 11;439(2):129-33. doi: 10.1016/j.neulet.2008.05.003. Epub 2008 May 7. Neurosci Lett. 2008. PMID: 18514411
-
Application of a C. elegans dopamine neuron degeneration assay for the validation of potential Parkinson's disease genes.J Vis Exp. 2008 Jul 18;(17):835. doi: 10.3791/835. J Vis Exp. 2008. PMID: 19066512 Free PMC article.
-
Disruption of dopamine homeostasis underlies selective neurodegeneration mediated by alpha-synuclein.Eur J Neurosci. 2007 Dec;26(11):3104-12. doi: 10.1111/j.1460-9568.2007.05929.x. Epub 2007 Nov 14. Eur J Neurosci. 2007. PMID: 18005066
-
The Caenorhabditis elegans dopaminergic system: opportunities for insights into dopamine transport and neurodegeneration.Annu Rev Pharmacol Toxicol. 2003;43:521-44. doi: 10.1146/annurev.pharmtox.43.100901.135934. Epub 2002 Jan 10. Annu Rev Pharmacol Toxicol. 2003. PMID: 12415122 Review.
-
A predictable worm: application of Caenorhabditis elegans for mechanistic investigation of movement disorders.Neurotherapeutics. 2012 Apr;9(2):393-404. doi: 10.1007/s13311-012-0109-x. Neurotherapeutics. 2012. PMID: 22403010 Free PMC article. Review.
Cited by
-
Interaction of genetic and environmental factors in a Drosophila parkinsonism model.J Neurosci. 2007 Mar 7;27(10):2457-67. doi: 10.1523/JNEUROSCI.4239-06.2007. J Neurosci. 2007. PMID: 17344383 Free PMC article.
-
Systemic RNA Interference Defective (SID) genes modulate dopaminergic neurodegeneration in C. elegans.PLoS Genet. 2022 Aug 19;18(8):e1010115. doi: 10.1371/journal.pgen.1010115. eCollection 2022 Aug. PLoS Genet. 2022. PMID: 35984862 Free PMC article.
-
Found in Translation: The Utility of C. elegans Alpha-Synuclein Models of Parkinson's Disease.Brain Sci. 2019 Mar 28;9(4):73. doi: 10.3390/brainsci9040073. Brain Sci. 2019. PMID: 30925741 Free PMC article. Review.
-
Potentiated Hsp104 variants antagonize diverse proteotoxic misfolding events.Cell. 2014 Jan 16;156(1-2):170-82. doi: 10.1016/j.cell.2013.11.047. Cell. 2014. PMID: 24439375 Free PMC article.
-
Chronic exposure to methylmercury induces puncta formation in cephalic dopaminergic neurons in Caenorhabditis elegans.Neurotoxicology. 2020 Mar;77:105-113. doi: 10.1016/j.neuro.2020.01.003. Epub 2020 Jan 11. Neurotoxicology. 2020. PMID: 31935438 Free PMC article.
References
-
- Augood SJ, Hollingworth Z, Albers DS, Yang L, Leung JC, Muller B, Klein C, Breakefield XO, Standaert DG (2002) Dopamine transmission in DYT1 dystonia: a biochemical and autoradiographical study. Neurology 59: 445-448. - PubMed
-
- Augood SJ, Hollingworth Z, Albers DS, Yang L, Leung J, Breakefield XO, Standaert DG (2004) Dopamine transmission in DYT1 dystonia. Adv Neurol 94: 53-60. - PubMed
-
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM (2002) Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science 295: 809-810. - PubMed
-
- Berger K, Przedborski S, Cadet JL (1991) Retrograde degeneration of nigrostriatal neurons induced by intrastriatal 6-hydroxydopamine injection in rats. Brain Res Bull 26: 301-307. - PubMed
-
- Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM (2001) Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease. Prog Neurobiol 65: 135-172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
