Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II)
- PMID: 15829642
- PMCID: PMC6724918
- DOI: 10.1523/JNEUROSCI.0102-05.2005
Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II)
Abstract
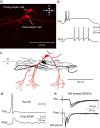
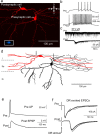
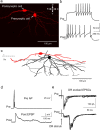
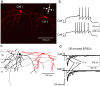
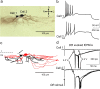
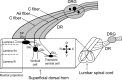
Neural circuitry of the spinal superficial dorsal horn (SDH) (laminae I and II) and its relationship to pain and other somatosensory phenomena remain poorly understood. To gain information on this issue, synaptic connections between identified SDH neurons were studied in rat spinal cord slices by simultaneous whole-cell recordings from pairs of cells. Both excitatory and inhibitory connections were noted. This report focuses on the observed excitatory linkages. Synaptic excitatory connections between SDH neurons proved highly selective and consistently were unidirectional. Two patterns repeatedly appeared (for neuron classification, see Materials and Methods) (Grudt and Perl, 2002). Lamina II central neurons, with dorsal root (DR) C-fiber input, monosynaptically excited lamina II vertical neurons with DR Adelta input. Lamina II outer vertical neurons with DR Adelta input monosynaptically excited lamina I neurons. Some of the postsynaptic lamina I cells were shown to project rostrally. In contrast to the usual case for connected neurons, in unconnected pairs, primary afferent input to the same type of neuron proved closely similar. Together, these observations indicate that the neural circuitry in the SDH, including its substantia gelatinosa (lamina II), has an explicit organization in which particular combinations of neurons comprise modules arranged to modify and transmit sensory information arriving from Adelta and C primary afferent fibers.
Figures

References
-
- Bennett GJ, Abdelmoumene M, Hayashi H, Dubner R (1980) Physiology and morphology of substantia gelatinosa neurons intracellularly stained with horseradish peroxidase. J Comp Neurol 194: 809-827. - PubMed
-
- Craig AD (1994) Spinal and supraspinal processing of specific pain and temperature. In: Touch, temperature, and pain in health and disease: mechanisms and assessments (Boivie J, Hansson P, Lindblom U, eds), pp 421-437. Seattle: International Association for the Study of Pain.
-
- Craig AD, Kniffki KD (1985) The multiple representation of nociception in the spinothalamic projection of lamina I cells in the cat. In: Development, organization, and processing in somatosensory pathways (Rowe M, Willis W, eds), pp 347-353. New York: Alan R. Liss.
-
- Earle KM (1952) The tract of Lissauer and its possible relation to the pain pathway. J Comp Neurol 96: 93-109. - PubMed
-
- Gobel S (1978) Golgi studies of the neurons in layer II of the dorsal horn of the medulla (trigeminal nucleus caudalis). J Comp Neurol 180: 395-414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
