Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis
- PMID: 15831232
- PMCID: PMC1871894
- DOI: 10.1215/S1152851704001115
Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis
Abstract
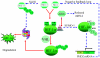
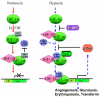
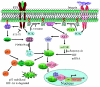
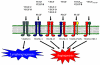
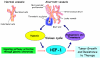
Glioblastomas, like other solid tumors, have extensive areas of hypoxia and necrosis. The importance of hypoxia in driving tumor growth is receiving increased attention. Hypoxia-inducible factor 1 (HIF-1) is one of the master regulators that orchestrate the cellular responses to hypoxia. It is a heterodimeric transcription factor composed of alpha and beta subunits. The alpha subunit is stable in hypoxic conditions but is rapidly degraded in normoxia. The function of HIF-1 is also modulated by several molecular mechanisms that regulate its synthesis, degradation, and transcriptional activity. Upon stabilization or activation, HIF-1 translocates to the nucleus and induces transcription of its downstream target genes. Most important to gliomagenesis, HIF-1 is a potent activator of angiogenesis and invasion through its upregulation of target genes critical for these functions. Activation of the HIF-1 pathway is a common feature of gliomas and may explain the intense vascular hyperplasia often seen in glioblastoma multiforme. Activation of HIF results in the activation of vascular endothelial growth factors, vascular endothelial growth factor receptors, matrix metalloproteinases, plasminogen activator inhibitor, transforming growth factors alpha and beta, angiopoietin and Tie receptors, endothelin-1, inducible nitric oxide synthase, adrenomedullin, and erythropoietin, which all affect glioma angiogenesis. In conclusion, HIF is a critical regulatory factor in the tumor microenvironment because of its central role in promoting proangiogenic and invasive properties. While HIF activation strongly promotes angiogenesis, the emerging vasculature is often abnormal, leading to a vicious cycle that causes further hypoxia and HIF upregulation.
Figures

References
-
- Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–38465. - PubMed
-
- Asano T, Aoyagi M, Hirakawa K, Ikawa Y. Effect of endothelin-1 as growth factor on a human glioma cell line; its characteristic promotion of DNA synthesis. J Neurooncol. 1994;18:1–7. - PubMed
-
- Bagnato A, Spinella F. Emerging role of endothelin-1 in tumor angiogenesis. Trends Endocrinol Metab. 2003;14:44–50. - PubMed
-
- Bajou K, Maillard C, Jost M, Lijnen RH, Gils A, Declerck P, Carmeliet P, Foidart JM, Noel A. Host-derived plasminogen activator inhibitor-1 (PAI-1) concentration is critical for in vivo tumoral angiogenesis and growth. Oncogene. 2004;23:6986–6990. - PubMed
-
- Barker FG, 2nd, Davis RL, Chang SM, Prados MD. Necrosis as a prognostic factor in glioblastoma multiforme. Cancer. 1996;77:1161–1166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

