Integrin-linked kinase mediates bone morphogenetic protein 7-dependent renal epithelial cell morphogenesis
- PMID: 15831470
- PMCID: PMC1084303
- DOI: 10.1128/MCB.25.9.3648-3657.2005
Integrin-linked kinase mediates bone morphogenetic protein 7-dependent renal epithelial cell morphogenesis
Abstract
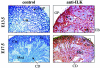
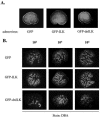
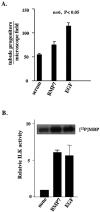
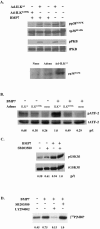
Bone morphogenetic protein 7 (BMP7) stimulates renal branching morphogenesis via p38 mitogen-activated protein kinase (p38(MAPK)) and activating transcription factor 2 (ATF-2) (M. C. Hu, D. Wasserman, S. Hartwig, and N. D. Rosenblum, J. Biol. Chem. 279:12051-12059, 2004). Here, we demonstrate a novel role for integrin-linked kinase (ILK) in mediating renal epithelial cell morphogenesis in embryonic kidney explants and identify p38(MAPK) as a target of ILK signaling in a cell culture model of renal epithelial morphogenesis. The spatial and temporal expression of ILK in embryonic mouse kidney cells suggested a role in branching morphogenesis. Adenovirus-mediated expression of ILK stimulated and expression of a dominant negative ILK mutant inhibited ureteric bud branching in embryonic mouse kidney explants. BMP7 increased ILK kinase activity in inner medullary collecting duct 3 (IMCD-3) cells, and adenovirus-mediated expression of ILK increased IMCD-3 cell morphogenesis in a three-dimensional culture model. In contrast, treatment with a small molecule ILK inhibitor or expression of a dominant negative-acting ILK (ILK(E359K)) inhibited epithelial cell morphogenesis. Further, expression of ILK(E359K) abrogated BMP7-dependent stimulation. To investigate the role of ILK in BMP7 signaling, we showed that ILK overexpression increased basal and BMP7-induced levels of phospho-p38(MAPK) and phospho-ATF-2. Consistent with its inhibitory effects on IMCD-3 cell morphogenesis, expression of ILK(E359K) blocked BMP7-dependent increases in phospho-p38(MAPK) and phospho-ATF-2. Inhibition of p38(MAPK) activity with the specific inhibitor, SB203580, failed to inhibit BMP7-dependent stimulation of ILK activity, suggesting that ILK functions upstream of p38(MAPK) during BMP7 signaling. We conclude that ILK functions in a BMP7/p38(MAPK)/ATF-2 signaling pathway and stimulates epithelial cell morphogenesis.
Figures



Similar articles
-
p38MAPK acts in the BMP7-dependent stimulatory pathway during epithelial cell morphogenesis and is regulated by Smad1.J Biol Chem. 2004 Mar 26;279(13):12051-9. doi: 10.1074/jbc.M310526200. Epub 2004 Jan 12. J Biol Chem. 2004. PMID: 14718543
-
Integrin-linked Kinase Controls Renal Branching Morphogenesis via Dual Specificity Phosphatase 8.J Am Soc Nephrol. 2016 May;27(5):1465-77. doi: 10.1681/ASN.2015020139. Epub 2015 Sep 25. J Am Soc Nephrol. 2016. PMID: 26407593 Free PMC article.
-
Regulation of GDF-8 signaling by the p38 MAPK.Cell Signal. 2005 Mar;17(3):365-75. doi: 10.1016/j.cellsig.2004.08.003. Cell Signal. 2005. PMID: 15567067
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
Bone morphogenetic protein-7 (BMP7) in chronic kidney disease.Front Biosci. 2008 May 1;13:4726-39. doi: 10.2741/3035. Front Biosci. 2008. PMID: 18508541 Review.
Cited by
-
Identification and characterization of a novel integrin-linked kinase inhibitor.J Med Chem. 2011 Sep 22;54(18):6364-74. doi: 10.1021/jm2007744. Epub 2011 Aug 24. J Med Chem. 2011. PMID: 21823616 Free PMC article.
-
Expression of focal adhesion proteins in the developing rat kidney.J Histochem Cytochem. 2011 Sep;59(9):864-74. doi: 10.1369/0022155411413929. Epub 2011 Jun 24. J Histochem Cytochem. 2011. PMID: 21705647 Free PMC article.
-
Cellular Recruitment by Podocyte-Derived Pro-migratory Factors in Assembly of the Human Renal Filter.iScience. 2019 Oct 25;20:402-414. doi: 10.1016/j.isci.2019.09.029. Epub 2019 Sep 26. iScience. 2019. PMID: 31622881 Free PMC article.
-
Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression.Cancer Sci. 2007 Oct;98(10):1512-20. doi: 10.1111/j.1349-7006.2007.00550.x. Epub 2007 Jul 23. Cancer Sci. 2007. PMID: 17645776 Free PMC article. Review.
-
BMP7 dose-dependently stimulates proliferation and cadherin-11 expression via ERK and p38 in a murine metanephric mesenchymal cell line.Physiol Rep. 2017 Aug;5(16):e13378. doi: 10.14814/phy2.13378. Physiol Rep. 2017. PMID: 28867673 Free PMC article.
References
-
- Bernardini, N., F. Bianchi, and A. Dolfi. 1999. Laminin and beta1 integrin distribution in the early stages of human kidney development. Nephron 81:289-295. - PubMed
-
- Campana, W. M., R. R. Myers, and A. Rearden. 2003. Identification of PINCH in Schwann cells and DRG neurons: shuttling and signaling after nerve injury. Glia 41:213-223. - PubMed
-
- Cantley, L. G., E. J. Barros, M. Gandhi, M. Rauchman, and S. K. Nigam. 1994. Regulation of mitogenesis, motogenesis, and tubulogenesis by hepatocyte growth factor in renal collecting duct cells. Am. J. Physiol. 267:F271-F280. - PubMed
-
- D'Amico, M., J. Hulit, D. F. Amanatullah, B. T. Zafonte, C. Albanese, B. Bouzahzah, M. Fu, L. H. Augenlicht, L. A. Donehower, K. Takemaru, R. T. Moon, R. Davis, M. P. Lisanti, M. Shtutman, J. Zhurinsky, A. Ben-Ze'ev, A. A. Troussard, S. Dedhar, and R. G. Pestell. 2000. The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3β and cAMP-responsive element-binding protein-dependent pathways. J. Biol. Chem. 275:32649-32657. - PubMed
-
- Davies, J. A., and C. E. Fisher. 2002. Genes and proteins in renal development. Exp. Nephrol. 10:102-113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
