The human stress-activated protein kin17 belongs to the multiprotein DNA replication complex and associates in vivo with mammalian replication origins
- PMID: 15831485
- PMCID: PMC1084281
- DOI: 10.1128/MCB.25.9.3814-3830.2005
The human stress-activated protein kin17 belongs to the multiprotein DNA replication complex and associates in vivo with mammalian replication origins
Abstract
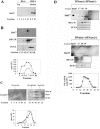
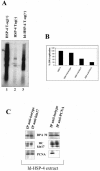
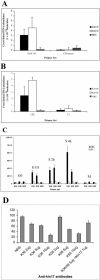
The human stress-activated protein kin17 accumulates in the nuclei of proliferating cells with predominant colocalization with sites of active DNA replication. The distribution of kin17 protein is in equilibrium between chromatin-DNA and the nuclear matrix. An increased association with nonchromatin nuclear structure is observed in S-phase cells. We demonstrated here that kin17 protein strongly associates in vivo with DNA fragments containing replication origins in both human HeLa and monkey CV-1 cells. This association was 10-fold higher than that observed with nonorigin control DNA fragments in exponentially growing cells. In addition, the association of kin17 protein to DNA fragments containing replication origins was also analyzed as a function of the cell cycle. High binding of kin17 protein was found at the G(1)/S border and throughout the S phase and was negligible in both G(0) and M phases. Specific monoclonal antibodies against kin17 protein induced a threefold inhibition of in vitro DNA replication of a plasmid containing a minimal replication origin that could be partially restored by the addition of recombinant kin17 protein. Immunoelectron microscopy confirmed the colocalization of kin17 protein with replication proteins like RPA, PCNA, and DNA polymerase alpha. A two-step chromatographic fractionation of nuclear extracts from HeLa cells revealed that kin17 protein localized in vivo in distinct protein complexes of high molecular weight. We found that kin17 protein purified within an approximately 600-kDa protein complex able to support in vitro DNA replication by means of two different biochemical methods designed to isolate replication complexes. In addition, the reduced in vitro DNA replication activity of the multiprotein replication complex after immunodepletion for kin17 protein highlighted for a direct role in DNA replication at the origins.
Figures




Similar articles
-
Participation of kin17 protein in replication factories and in other DNA transactions mediated by high molecular weight nuclear complexes.Mol Cancer Res. 2003 May;1(7):519-31. Mol Cancer Res. 2003. PMID: 12754299
-
Selective interactions of human kin17 and RPA proteins with chromatin and the nuclear matrix in a DNA damage- and cell cycle-regulated manner.Nucleic Acids Res. 2003 Jul 15;31(14):4162-75. doi: 10.1093/nar/gkg459. Nucleic Acids Res. 2003. PMID: 12853634 Free PMC article.
-
Human kin17 protein directly interacts with the simian virus 40 large T antigen and inhibits DNA replication.Cancer Res. 2002 Oct 1;62(19):5425-35. Cancer Res. 2002. PMID: 12359749
-
Dynamics of pre-replication complex proteins during the cell division cycle.Philos Trans R Soc Lond B Biol Sci. 2004 Jan 29;359(1441):7-16. doi: 10.1098/rstb.2003.1360. Philos Trans R Soc Lond B Biol Sci. 2004. PMID: 15065651 Free PMC article. Review.
-
Eukaryotic DNA replication: Orchestrated action of multi-subunit protein complexes.Mutat Res. 2018 May;809:58-69. doi: 10.1016/j.mrfmmm.2017.04.002. Epub 2017 May 1. Mutat Res. 2018. PMID: 28501329 Review.
Cited by
-
A genetic screen in C. elegans reveals roles for KIN17 and PRCC in maintaining 5' splice site identity.PLoS Genet. 2022 Feb 10;18(2):e1010028. doi: 10.1371/journal.pgen.1010028. eCollection 2022 Feb. PLoS Genet. 2022. PMID: 35143478 Free PMC article.
-
Deficiency of kin17 Facilitates Apoptosis of Cervical Cancer Cells by Modulating Caspase 3, PARP, and Bcl-2 Family Proteins.J Oncol. 2022 Jul 20;2022:3156968. doi: 10.1155/2022/3156968. eCollection 2022. J Oncol. 2022. PMID: 35909901 Free PMC article.
-
A conserved KIN17 curved DNA-binding domain protein assembles with SQUAMOSA PROMOTER-BINDING PROTEIN-LIKE7 to adapt Arabidopsis growth and development to limiting copper availability.Plant Physiol. 2014 Feb;164(2):828-40. doi: 10.1104/pp.113.228239. Epub 2013 Dec 13. Plant Physiol. 2014. PMID: 24335506 Free PMC article.
-
Expression of Kin17 promotes the proliferation of hepatocellular carcinoma cells in vitro and in vivo.Oncol Lett. 2014 Sep;8(3):1190-1194. doi: 10.3892/ol.2014.2244. Epub 2014 Jun 12. Oncol Lett. 2014. PMID: 25120685 Free PMC article.
-
Onvansertib and Navitoclax Combination as a New Therapeutic Option for Mucinous Ovarian Carcinoma.Int J Mol Sci. 2025 Jan 8;26(2):472. doi: 10.3390/ijms26020472. Int J Mol Sci. 2025. PMID: 39859203 Free PMC article.
References
-
- Alvarez, D., M. Callejo, R. Shoucri, L. Boyer, G. B. Price, and M. Zannis-Hadjopoulos. 2003. Analysis of the cruciform binding activity of recombinant 14-3-3ζ-MBP fusion protein, its heterodimerization profile with endogenous 14-3-3 isoforms, and effect on mammalian DNA replication in vitro. Biochemistry 42:7205-7215. - PubMed
-
- Araujo, F. D., J. D. Knox, S. Ramchandani, R. Pelletier, P. Bigey, G. Price, M. Szyf, and M. Zannis-Hadjopoulos. 1999. Identification of initiation sites for DNA replication in the human dnmt1 (DNA-methyltransferase) locus. J. Biol. Chem. 274:9335-9341. - PubMed
-
- Batty, D. P., and R. D. Wood. 2000. Damage recognition in nucleotide excision repair of DNA. Gene 241:193-204. - PubMed
-
- Berezney, R., M. J. Mortillaro, H. Ma, X. Wei, and J. Samarabandu. 1995. The nuclear matrix: a structural milieu for genomic function. Int. Rev. Cytol. 162A:1-65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
