Proteasomal ATPase-associated factor 1 negatively regulates proteasome activity by interacting with proteasomal ATPases
- PMID: 15831487
- PMCID: PMC1084299
- DOI: 10.1128/MCB.25.9.3842-3853.2005
Proteasomal ATPase-associated factor 1 negatively regulates proteasome activity by interacting with proteasomal ATPases
Abstract
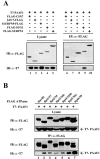
The 26S proteasome, composed of the 20S core and the 19S regulatory complex, plays a central role in ubiquitin-dependent proteolysis by catalyzing degradation of polyubiquitinated proteins. In a search for proteins involved in regulation of the proteasome, we affinity purified the 19S regulatory complex from HeLa cells and identified a novel protein of 43 kDa in size as an associated protein. Immunoprecipitation analyses suggested that this protein specifically interacted with the proteasomal ATPases. Hence the protein was named proteasomal ATPase-associated factor 1 (PAAF1). Immunoaffinity purification of PAAF1 confirmed its interaction with the 19S regulatory complex and further showed that the 19S regulatory complex bound with PAAF1 was not stably associated with the 20S core. Overexpression of PAAF1 in HeLa cells decreased the level of the 20S core associated with the 19S complex in a dose-dependent fashion, suggesting that PAAF1 binding to proteasomal ATPases inhibited the assembly of the 26S proteasome. Proteasomal degradation assays using reporters based on green fluorescent protein revealed that overexpression of PAAF1 inhibited the proteasome activity in vivo. Furthermore, the suppression of PAAF1 expression that is mediated by small inhibitory RNA enhanced the proteasome activity. These results suggest that PAAF1 functions as a negative regulator of the proteasome by controlling the assembly/disassembly of the proteasome.
Figures









Similar articles
-
The proteasome regulates HIV-1 transcription by both proteolytic and nonproteolytic mechanisms.Mol Cell. 2007 Feb 9;25(3):369-83. doi: 10.1016/j.molcel.2006.12.020. Mol Cell. 2007. PMID: 17289585
-
The 19S ATPase S6a (S6'/TBP1) regulates the transcription initiation of class II transactivator.J Mol Biol. 2010 Jan 15;395(2):254-69. doi: 10.1016/j.jmb.2009.10.035. Epub 2009 Oct 21. J Mol Biol. 2010. PMID: 19853614
-
Tissue and cell distribution of a mammalian proteasomal ATPase, MSS1, and its complex formation with the basal transcription factors.Biochem Biophys Res Commun. 2000 Dec 20;279(2):568-73. doi: 10.1006/bbrc.2000.3969. Biochem Biophys Res Commun. 2000. PMID: 11118327
-
Proteasomes and their associated ATPases: a destructive combination.J Struct Biol. 2006 Oct;156(1):72-83. doi: 10.1016/j.jsb.2006.04.012. Epub 2006 May 8. J Struct Biol. 2006. PMID: 16919475 Review.
-
Order of the proteasomal ATPases and eukaryotic proteasome assembly.Cell Biochem Biophys. 2011 Jun;60(1-2):13-20. doi: 10.1007/s12013-011-9178-4. Cell Biochem Biophys. 2011. PMID: 21461838 Free PMC article. Review.
Cited by
-
PPI network analyses of human WD40 protein family systematically reveal their tendency to assemble complexes and facilitate the complex predictions.BMC Syst Biol. 2018 Apr 24;12(Suppl 4):41. doi: 10.1186/s12918-018-0567-9. BMC Syst Biol. 2018. PMID: 29745845 Free PMC article.
-
Multiple assembly chaperones govern biogenesis of the proteasome regulatory particle base.Cell. 2009 May 29;137(5):887-99. doi: 10.1016/j.cell.2009.04.061. Epub 2009 May 14. Cell. 2009. PMID: 19446322 Free PMC article.
-
Spt6 levels are modulated by PAAF1 and proteasome to regulate the HIV-1 LTR.Retrovirology. 2012 Feb 8;9:13. doi: 10.1186/1742-4690-9-13. Retrovirology. 2012. PMID: 22316138 Free PMC article.
-
Proteasome regulation by ADP-ribosylation.Cell. 2013 Apr 25;153(3):614-27. doi: 10.1016/j.cell.2013.03.040. Cell. 2013. PMID: 23622245 Free PMC article.
-
Subcomplexes of PA700, the 19 S regulator of the 26 S proteasome, reveal relative roles of AAA subunits in 26 S proteasome assembly and activation and ATPase activity.J Biol Chem. 2009 Sep 11;284(37):24891-903. doi: 10.1074/jbc.M109.023218. Epub 2009 Jul 9. J Biol Chem. 2009. PMID: 19589775 Free PMC article.
References
-
- Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. - PubMed
-
- Dantuma, N. P., K. Lindsten, R. Glas, M. Jellne, and M. G. Masucci. 2000. Short-lived green fluorescent proteins for quantifying ubiquitin/proteasome-dependent proteolysis in living cells. Nat. Biotechnol. 18:538-543. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
