Ab initio molecular dynamics and quasichemical study of H+(aq)
- PMID: 15831590
- PMCID: PMC1100742
- DOI: 10.1073/pnas.0408071102
Ab initio molecular dynamics and quasichemical study of H+(aq)
Abstract
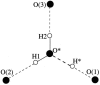
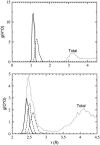
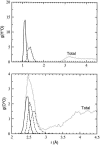
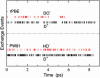
The excess proton in water, H(+)(aq), plays a fundamental role in aqueous solution chemistry. Its solution thermodynamic properties are essential to molecular descriptions of that chemistry and for validation of dynamical calculations. Within the quasichemical theory of solutions those thermodynamic properties are conditional on recognizing underlying solution structures. The quasichemical treatment identifies H(3)O(+) and H(2)O(5)(+) as natural inner-shell complexes, corresponding to the cases of n = 1, 2 water molecule ligands, respectively, of a distinguished H(+) ion. A quantum-mechanical treatment of the inner-shell complex with both a dielectric continuum and a classical molecular dynamics treatment of the outer-shell contribution identifies the latter case (the Zundel complex) as the more numerous species. Ab initio molecular dynamics simulations, with two different electron density functionals, suggest a preponderance of Zundel-like structures, but a symmetrical ideal Zundel cation is not observed.
Figures
References
-
- Asthagiri, D., Pratt, L. R., Paulaitis, M. E. & Rempe, S. B. (2004) J. Am. Chem. Soc. 126, 1285–1289. - PubMed
-
- Liu, W. B., Sakane, S., Wood, R. H. & Doren, D. J. (2002) J. Phys. Chem. A 106, 1409–1418.
-
- Grabowski, P., Riccardi, D., Gomez, M. A., Asthagiri, D. & Pratt, L. R. (2002) J. Phys. Chem. A 106, 9145–9148.
-
- Liu, W. B., Wood, R. H. & Doren, D. J. (2003) J. Chem. Phys. 118, 2837–2844.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

