Inhibitors of mitogen-activated protein kinases differentially regulate costimulated T cell cytokine production and mouse airway eosinophilia
- PMID: 15833106
- PMCID: PMC1131927
- DOI: 10.1186/1465-9921-6-36
Inhibitors of mitogen-activated protein kinases differentially regulate costimulated T cell cytokine production and mouse airway eosinophilia
Abstract
Background: T cells play a dominant role in the pathogenesis of asthma. Costimulation of T cells is necessary to fully activate them. An inducible costimulator (ICOS) of T cells is predominantly expressed on Th2 cells. Therefore, interference of signaling pathways precipitated by ICOS may present new therapeutic options for Th2 dominated diseases such as asthma. However, these signaling pathways are poorly characterized in vitro and in vivo.
Methods: Human primary CD4+ T cells from blood were activated by beads with defined combinations of surface receptor stimulating antibodies and costimulatory receptor ligands. Real-time RT-PCR was used for measuring the production of cytokines from activated T cells. Activation of mitogen activated protein kinase (MAPK) signaling pathways leading to cytokine synthesis were investigated by western blot analysis and by specific inhibitors. The effect of inhibitors in vivo was tested in a murine asthma model of late phase eosinophilia. Lung inflammation was assessed by differential cell count of the bronchoalveolar lavage, determination of serum IgE and lung histology.
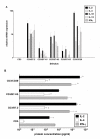
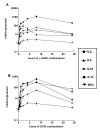
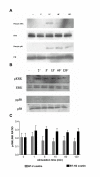
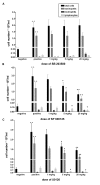
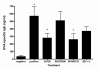
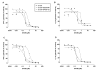
Results: We showed in vitro that ICOS and CD28 are stimulatory members of an expanding family of co-receptors, whereas PD1 ligands failed to co-stimulate T cells. ICOS and CD28 activated different MAPK signaling cascades necessary for cytokine activation. By means of specific inhibitors we showed that p38 and ERK act downstream of CD28 and that ERK and JNK act downstream of ICOS leading to the induction of various T cell derived cytokines. Using a murine asthma model of late phase eosinophilia, we demonstrated that the ERK inhibitor U0126 and the JNK inhibitor SP600125 inhibited lung inflammation in vivo. This inhibition correlated with the inhibition of Th2 cytokines in the BAL fluid. Despite acting on different signaling cascades, we could not detect synergistic action of any combination of MAPK inhibitors. In contrast, we found that the p38 inhibitor SB203580 antagonizes the action of the ERK inhibitor U0126 in vitro and in vivo.
Conclusion: These results demonstrate that the MAPKs ERK and JNK may be suitable targets for anti-inflammatory therapy of asthma, whereas inhibition of p38 seems to be an unlikely target.
Figures



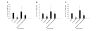
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

