The evolving clinical profile of abatacept (CTLA4-Ig): a novel co-stimulatory modulator for the treatment of rheumatoid arthritis
- PMID: 15833145
- PMCID: PMC2833980
- DOI: 10.1186/ar1688
The evolving clinical profile of abatacept (CTLA4-Ig): a novel co-stimulatory modulator for the treatment of rheumatoid arthritis
Abstract
Abatacept (CTLA4-Ig) is a novel fusion protein designed to modulate the T cell co-stimulatory signal mediated through the CD28-CD80/86 pathway. Clinical trials have provided preliminary evidence of the efficacy of this compound in the treatment of rheumatoid arthritis. This review describes the molecular and biologic bases for the use of abatacept in rheumatoid arthritis and summarizes the current clinical data on its safety and effectiveness in this disease.
Figures
Similar articles
-
Abatacept, a novel CD80/86-CD28 T cell co-stimulation modulator, in the treatment of rheumatoid arthritis.Basic Clin Pharmacol Toxicol. 2009 Apr;104(4):276-84. doi: 10.1111/j.1742-7843.2009.00375.x. Epub 2009 Feb 18. Basic Clin Pharmacol Toxicol. 2009. PMID: 19228144 Review.
-
Abatacept mechanism of action: concordance with its clinical profile.Reumatol Clin. 2012 Mar-Apr;8(2):78-83. doi: 10.1016/j.reuma.2011.08.002. Epub 2011 Nov 21. Reumatol Clin. 2012. PMID: 22104048 Review.
-
Technology evaluation: abatacept, Bristol-Myers Squibb.Curr Opin Mol Ther. 2004 Jun;6(3):318-30. Curr Opin Mol Ther. 2004. PMID: 15264435 Review.
-
Role of abatacept in the management of rheumatoid arthritis.Clin Ther. 2006 Nov;28(11):1764-78. doi: 10.1016/j.clinthera.2006.11.020. Clin Ther. 2006. PMID: 17212998 Review.
-
T cells of staphylococcal enterotoxin B-tolerized autoimmune MRL-lpr/lpr mice require co-stimulation through the B7-CD28/CTLA-4 pathway for activation and can be reanergized in vivo by stimulation of the T cell receptor in the absence of this co-stimulatory signal.Eur J Immunol. 1994 May;24(5):1019-25. doi: 10.1002/eji.1830240502. Eur J Immunol. 1994. PMID: 7514125
Cited by
-
Current and future therapies for primary Sjögren syndrome.Nat Rev Rheumatol. 2021 Aug;17(8):475-486. doi: 10.1038/s41584-021-00634-x. Epub 2021 Jun 29. Nat Rev Rheumatol. 2021. PMID: 34188206 Review.
-
Leveraging human genetics to develop future therapeutic strategies in rheumatoid arthritis.Rheum Dis Clin North Am. 2010 May;36(2):259-70. doi: 10.1016/j.rdc.2010.03.002. Rheum Dis Clin North Am. 2010. PMID: 20510233 Free PMC article. Review.
-
Tolerogenic Dendritic Cells on Transplantation: Immunotherapy Based on Second Signal Blockage.J Immunol Res. 2015;2015:856707. doi: 10.1155/2015/856707. Epub 2015 Oct 12. J Immunol Res. 2015. PMID: 26543876 Free PMC article. Review.
-
The pathogenesis and treatment in antineutrophil cytoplasmic antibody associated vasculitis.Am J Transl Res. 2020 Aug 15;12(8):4094-4107. eCollection 2020. Am J Transl Res. 2020. PMID: 32913491 Free PMC article. Review.
-
Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand.Proc Natl Acad Sci U S A. 2011 Jan 11;108(2):768-73. doi: 10.1073/pnas.1013492108. Epub 2010 Dec 27. Proc Natl Acad Sci U S A. 2011. PMID: 21187391 Free PMC article.
References
-
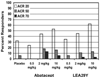
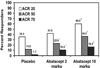
- Moreland LW, Alten R, Van den Bosch F, Appelboom T, Leon M, Emery P, Cohen S, Luggen M, Shergy W, Nuamah I. Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose-finding, double-blind, placebo-controlled clinical trial evaluating CTLA-4Ig and LEA29Y eighty-five days after the first infusion. Arthritis Rheum. 2002;46:1470–1479. doi: 10.1002/art.10294. - DOI - PubMed
-
- Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991;147:2461–2466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

