Pervasive regulation of Drosophila Notch target genes by GY-box-, Brd-box-, and K-box-class microRNAs
- PMID: 15833912
- PMCID: PMC1091741
- DOI: 10.1101/gad.1291905
Pervasive regulation of Drosophila Notch target genes by GY-box-, Brd-box-, and K-box-class microRNAs
Abstract
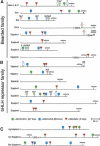
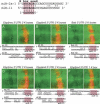
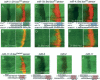
Although hundreds of distinct animal microRNAs (miRNAs) are known, the specific biological functions of only a handful are understood at present. Here, we demonstrate that three different families of Drosophila miRNAs directly regulate two large families of Notch target genes, including basic helix-loop-helix (bHLH) repressor and Bearded family genes. These miRNAs regulate Notch target gene activity via GY-box (GUCUUCC), Brd-box (AGCUUUA), and K-box (cUGUGAUa) motifs. These are conserved sites in target 3'-untranslated regions (3'-UTRs) that are complementary to the 5'-ends of miRNAs, or "seed" regions. Collectively, these motifs represent >40 miRNA-binding sites in Notch target genes, and we show all three classes of motif to be necessary and sufficient for miRNA-mediated regulation in vivo. Importantly, many of the validated miRNA-binding sites have limited pairing to miRNAs outside of the "box:seed" region. Consistent with this, we find that seed-related miRNAs that are otherwise quite divergent can regulate the same target sequences. Finally, we demonstrate that ectopic expression of several Notch-regulating miRNAs induces mutant phenotypes that are characteristic of Notch pathway loss of function, including loss of wing margin, thickened wing veins, increased bristle density, and tufted bristles. Collectively, these data establish insights into miRNA target recognition and demonstrate that the Notch signaling pathway is a major target of miRNA-mediated regulation in Drosophila.
Figures





References
-
- Abrahante J.E., Daul, A.L., Li, M., Volk, M.L., Tennessen, J.M., Miller, E.A., and Rougvie, A.E. 2003. The Caenorhabditis elegans hunchback-like gene lin-57/hbl-1 controls developmental time and is regulated by microRNAs. Dev. Cell 4: 625–637. - PubMed
-
- Aravin A., Lagos-Quintana, M., Yalcin, A., Zavolan, M., Marks, D., Snyder, B., Gaasterland, T., Meyer, J., and Tuschl, T. 2003. The small RNA profile during Drosophila melanogaster development. Dev. Cell 5: 337–350. - PubMed
-
- Bailey A.M. and Posakony, J.W. 1995. Suppressor of Hairless directly activates transcription of Enhancer of split Complex genes in response to Notch receptor activity. Genes & Dev. 9: 2609–2622. - PubMed
-
- Bartel D.P. 2004. MicroRNAs. Genomics, biogenesis, mechanism, and function. Cell 116: 281–297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
