Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint
- PMID: 15833913
- PMCID: PMC1091739
- DOI: 10.1101/gad.1301205
Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint
Abstract
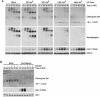
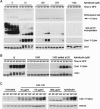
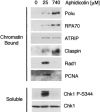
The ATR-dependent DNA damage response pathway can respond to a diverse group of lesions as well as inhibitors of DNA replication. Using the Xenopus egg extract system, we show that lesions induced by UV irradiation and cis-platinum cause the functional uncoupling of MCM helicase and DNA polymerase activities, an event previously shown for aphidicolin. Inhibition of uncoupling during elongation with inhibitors of MCM7 or Cdc45, a putative helicase cofactor, results in abrogation of Chk1 phosphorylation, indicating that uncoupling is necessary for activation of the checkpoint. However, uncoupling is not sufficient for checkpoint activation, and DNA synthesis by Polalpha is also required. Finally, using plasmids of varying size, we demonstrate that all of the unwound DNA generated at a stalled replication fork can contribute to the level of Chk1 phosphorylation, suggesting that uncoupling amplifies checkpoint signaling at each individual replication fork. Taken together, these observations indicate that functional uncoupling of MCM helicase and DNA polymerase activities occurs in response to multiple forms of DNA damage and that there is a general mechanism for generation of the checkpoint-activating signal following DNA damage.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
